David Spring and colleagues at University of Cambridge have developed a new methodology to rapidly screen large arrays of drug-like small molecules.
By using this new 3D microarray platform, they discover small molecules (novel pharmacores) that bind to quorum sensing receptors.
The platform has the potential to display and examine 10000 different small molecules in a miniaturised, low-cost format.
The authors say that ‘this new methodology is likely to find important application within the fields of medicinal chemistry, chemical biology, molecular recognition and chemical genetics, where the aim is to discover small molecules that bind to macromolecular complexes’
As a HOT article, it will be free to access until the 10th November.
Read more and tell us about it.
Discovery of a quorum sensing modulator pharmacophore by 3D small-molecule microarray screening
David M. Marsden, Rebecca L. Nicholson, Mette E. Skindersoe, Warren R. J. D. Galloway, Hannah F. Sore, Michael Givskov, George P. C. Salmond, Mark Ladlow, Martin Welch and David R. Spring
Org. Biomol. Chem., 2010, Advance Article
DOI: 10.1039/C0OB00300J, Paper













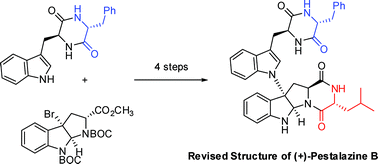
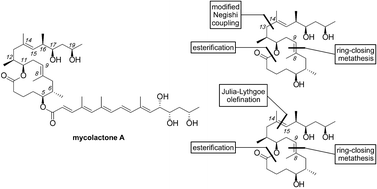 Synthetic studies on the mycolactone core
Synthetic studies on the mycolactone core 
 Hans-Joachim Knölkner and colleagues reveal the link between this beautiful church in Dresden and β-isocomene: silicon
Hans-Joachim Knölkner and colleagues reveal the link between this beautiful church in Dresden and β-isocomene: silicon