by Russell Johnson
A synthetic leaving group to be used in DNA synthesis could lead to new antiviral drugs or an artificial rival to DNA, say European scientists.
DNA can be synthesised using enzymes to catalyse the polymerisation of nucleoside triphosphate building blocks. Nucleoside monophosphates are incorporated in a growing nucleic acid chain, while pyrophosphates are released as leaving groups. Replacing the pyrophosphate leaving groups with synthetic alternatives could open up a range of biotech opportunities.
Now, Piet Herdewijn from the Catholic University of Leuven, Belgium, and colleagues have designed a series of leaving groups that are compatible with HIV-1 reverse transcriptase (an enzyme that synthesises DNA from RNA). They found that the most efficient pyrophosphate mimic was 3-phosphono-L-alanine. Herdewijn explains that they have managed to improve the kinetics of incorporating the nucleotide into the chain compared to previous leaving groups. However, 3-phosphono-L-alanine has a lower efficiency than pyrophosphate, which can cause DNA synthesis to stall. But the team says that they hope to use the stalling effect to create new antiviral drugs that selectively stall the synthesis of viral DNA, preventing virus replication.

The implications of this research aren’t limited to new drugs, says Herdewijn, and designing a replacement for the pyrophosphate group could be a step towards creating an artificial rival to DNA. ‘The long term goal is to look at nucleic acid synthesis using alternative biochemistry and eventually make xeno-nucleic acids [artificial nucleic acids] as an alternative to natural nucleic acids in vivo,’ explains Herdewijn.
David Jakeman, an expert in biosynthetic mechanisms at Dalhousie University, Canada, says that this could be an interesting avenue of research in the future. ‘The study of non-canonical mimics of pyrophosphate provides insight into the role of the leaving group in DNA synthesis, by HIV-1 reverse transcriptase. These advances may provide new directions for biotechnological applications of in vitro, and ultimately in vivo, DNA synthesis and replication.’
Read the full article here and the full story in Highlights in Chemical Biology












 Svetlana B Tsogoeva and colleagues at University of Erlagen-Nuremberg review the recent advances on the most successful C-C formation reactions using chiral BINOL phosphates as Bronsted acid catalysts.
Svetlana B Tsogoeva and colleagues at University of Erlagen-Nuremberg review the recent advances on the most successful C-C formation reactions using chiral BINOL phosphates as Bronsted acid catalysts.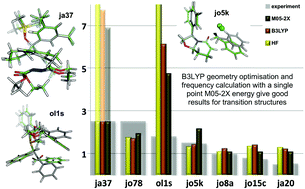 The manuscript reports an analysis of the performance of different density functionals in computational organic chemistry. The paper is based on a very large number of calculations and provides significant conclusions that have immediate application. The referees said that the manuscript stands out and that is why it is a HOT article.
The manuscript reports an analysis of the performance of different density functionals in computational organic chemistry. The paper is based on a very large number of calculations and provides significant conclusions that have immediate application. The referees said that the manuscript stands out and that is why it is a HOT article.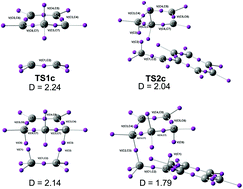
 Xianzhang Bu and colleagues at Sun Yat-Sen University in China have ‘unexpectedly’ discovered new fluorescent trans-dihydrofluoren-3-ones from one pot reactions of benzaldehydes and acetylacetone.
Xianzhang Bu and colleagues at Sun Yat-Sen University in China have ‘unexpectedly’ discovered new fluorescent trans-dihydrofluoren-3-ones from one pot reactions of benzaldehydes and acetylacetone.