 β-lactones are potentially useful as drugs for several diseases, due to their ability to inhibit key biological functions. However, their high reactivity in physiologically relevant conditions also renders their half-life very short and limits the potential drug applications.
β-lactones are potentially useful as drugs for several diseases, due to their ability to inhibit key biological functions. However, their high reactivity in physiologically relevant conditions also renders their half-life very short and limits the potential drug applications.
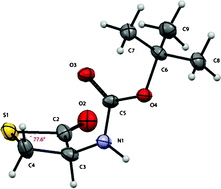
To combat this David Crich and colleagues at the Institut de Chimie des Substances Naturelles, CNRS, have synthesised a series of β-thiolactone analogs of β-lactones, on the basis that they would have greater persistence in aqueous media without too much loss of reactivity with the intended targets, as compared to β-lactam analogues. The synthesised β-thiolactones were assayed for cytotoxicity against several human cancer cell lines, where they showed greater activity than the corresponding lactones and lactams.
Interested? The article is free to access for 4 weeks:
Exploring the potential of the β-thiolactones in bioorganic chemistry
Sylvain Aubry, Kaname Sasaki, Laure Eloy, Geneviève Aubert, Pascal Retailleau, Thierry Cresteil and David Crich
Org. Biomol. Chem., 2011, Advance Article
DOI: 10.1039/C1OB05967J











![CoverIssue[4]](https://blogs.rsc.org/ob/files/2011/09/CoverIssue4.gif)