Scientists from Japan report on the use of thionium activation for nucleophilic functionalization of indole derivatives at the 2-alpha position.
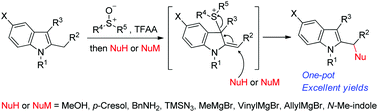
By using this method the indole ring is transformed into an allyl thionium species, and so is highly activated for nucleophilic attack from a wide variety of nucleophiles. Kawasaki make use of simple and readily available reagents, making the reaction a convenient route to functionalize alpha to the indole ring in a very straightforward fashion, enabling the introduction of O-, N- and C- substituents in a one-pot procedure.
For all the details read this paper for free today.
Sulfoxide-TFAA and nucleophile combination as new reagent for aliphatic C–H functionalization at indole 2α-position
Masanori Tayu, Kazuhiro Higuchi, Masato Inaba and Tomomi Kawasaki
DOI: 10.1039/C2OB26944A










