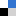Localized surface plasmon resonance (LSPR) are surface-localized, oscillating hot electrons generated by the interaction between light and plasmonic nanomaterials. Currently, the most widely used plasmonic materials are Au and Ag, which exhibit LSPR in the visible and near infrared region. If ultraviolet plasmonic materials were standardized, this would open up new possibilities in fields including LSPR catalysis and surface-enhanced Raman spectroscopy (SERS).
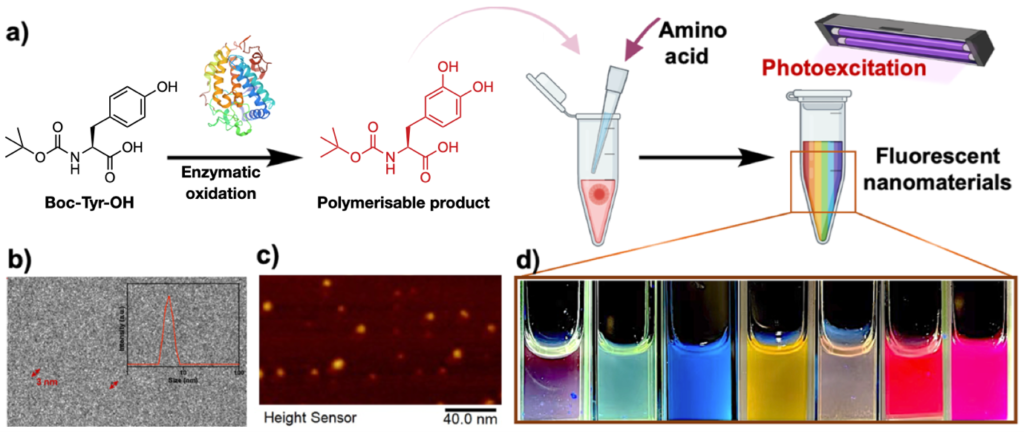
Recent work by a researchers from the University of Padova reported the synthesis of spherical Rh colloidal nanoparticles which sport well-defined LSPR in the ultraviolet region. Obtaining spherical Rh nanoparticles is challenging due to the preferred FCC crystal structure of Rh. Previous methods for the synthesis of spherical Rh nanoparticles were limited to the creation of particles with diameters within 7 nm, which only showed a weak plasmonic response. As shown in Fig. 1A-C, in this work, the researchers used laser ablation to create spherical Rh nanoparticles with diameters ranging between 20-45 nm. Moreover, since this approach of generating nanoparticles did not involve the use of any chemical ligands, this left the surface of the product particles accessible for the adsorption of ligand molecules which could be used to enhance colloidal stability or to provide additional functionality. To demonstrate this, the researchers functionalized the surface of the Rh nanoparticles with mercaptopropionic acid which interacted with metal ions, such as Cd (II), to induce agglomeration and in turn a change in the LSPR properties of the Rh colloid (Fig. 1D-E). As shown in Fig. 1F, the extent of agglomeration depended on the concentration of the metal ion, which allowed quantitative analysis of the concentration of metal ions in solution to be achieved. The surface accessibility of the Rh nanoparticles is also useful in facilitating the adsorption of analyte molecules for SERS analysis. By taking advantage of this property, the researchers demonstrated SERS detection of dyes, thiols and DNA using laser irradiation at 458 nm (Fig. 1G).
Fig 1 (A) Schematic illustrations of the synthesis of spherical Rh nanoparticles via laser ablation. (B-C) Transmission microscopy image of Rh nanoparticles ca. 45 nm in diameter and their LSPR response. (D) Schematic illustrations showing mercaptopropionic (MPA) functionalized Rh particles acting as LSPR optical sensors for metal ions. (E-F) The optical response of MPA-Rh colloids at different Cd(II) concentrations and for different metal ions in water. (G) SERS spectra of DNA, benzenethiol (BT), nitrothiolphenol (NTP), crystal violet (CV) and rhodamine B (RB) obtained using Rh nanoparticles as the enhancing substrate. Reproduced from DOI: 10.1039/d4nh00449c with permission from the Royal Society of Chemistry.
Another useful feature of the Rh nanoparticles is their improved stability in harsh conditions compared to Au and Ag nanoparticles, which for example could open new possibilities in operando SERS studies of catalytic processes that take place in pyrolysis. As shown in Fig. 2A, the Rh nanoparticles retained their structure when treated with aqua regia while Ag and Au nanoparticles were dissolved completely. Similarly, the Rh nanoparticles were found to be stable when heated to 500 ℃, while Au nanoparticles melted at this temperature (Fig. 2B).

Fig. 2 Scanning electron microscopy images of thiolated polyethylene glycol (PEG-SH) functionalized Rh and Au nanoparticles (NPs) before and after being treated with aqua regia (A) or 500 ℃ air (B). Reproduced from DOI: 10.1039/d4nh00449c with permission from the Royal Society of Chemistry.
In summary, spherical Rh colloidal nanoparticles with diameters ranging from 20-45 nm were created via laser ablation. Compared to conventional Au and Ag plasmonic nanoparticles, the Rh nanoparticles shown in this work exhibited well-defined LSPR in the ultraviolet region and high stability under harsh experimental conditions. This unique combination of properties broadens the applications of plasmonics and provides the tools for performing operando SERS studies in harsh conditions in which traditional plasmonic materials fail.
To find out more, please read:
Rhodium nanospheres for ultraviolet and visible plasmonics
David Muñeton Arboleda, Vito Coviello, Arianna Palumbo, Roberto Pilot and Vincenzo Amendola
Nanoscale Horiz., 2025, Advance Article
About the blogger

Yikai Xu is a tenure-track professor at East China University of Science and Technology. Before this he was a Leverhulme Early Career Fellow (PI) at Queen’s University Belfast. Dr Xu was the recipient of the 2019 Kathleen Lonsdale Royal Irish Academy Prize for the most outstanding PhD research in chemical science in Ireland. He is recognized as an “Emerging Investigator” by the Journal of Materials Chemistry C and Analyst. He currently serves as an Associate Editor for Carbon Capture Science & Technology, a Community Board member for Nanoscale Horizons and an Early Career Editor for Nano Materials Science. Dr Xu’s research interest lies in surface chemistry, SERS, and the bottom-up synthesis of surface-accessible plasmonic nanomaterials. |
Nanoparticle assembly with customisable fluorescence properties and excellent biocompatibility
Fluorescent reporters are invaluable tools for biomedical research like cell imaging, sensing or tracking analysis. In particular, the fluorescent labelling of nanomaterials remains a critical step in the development and evaluation of candidate nanomedicines. Being commercial fluorophores rather costly and fixed to a single emission, alternative strategies to produce labelled nanomaterials with tunable emission colour are highly coveted.
In a recent paper (DOI: 10.1039/d4nh00400k), Wang, Qi, et al. reported the versatile assembly of organic nanoparticles with adjustable emission wavelength by the enzymatic oxidation of the protected aminoacid N-(tert-butoxycarbonyl)-L-tyrosine. The biocatalytic oxidation of this aminoacid iduces its polymerisation into a variety of condensation products, which can co-assemble with unprotected aminoacids added post-polymerisation to generate nanoparticles ranging 5 to 10 nm in diameter. Interestingly, depending on the fed aminoacid post-polymerisation, the fluorescence spectra of the afforded nanoparticles could be shifted across the whole visible range. The fluorescent properties of these nanoparticles arise from the aggregation-induced emission of their constituent aminoacids, with different restrictions in bond rotation -and hence emission colour- for each nanoparticle formulation. Indeed, molecular dynamics simulations supported the aggregation mechanism and fixation of bond rotation, which together explain the assembly of these emissive nanoparticles.
The authors also demonstrated the excellent biocompatibility of these nanostructures in vitro and tracked their uptake by HeLa cells by confocal laser scanning microscopy. These results prove the great potential of this versatile technology to produce nanoparticles for biomedicine with tailored fluorescence from biomolecular precursors.
Overall, this paper lays down the basis for a new nanoparticle assembly platform with customisable fluorescence properties and excellent biocompatibility. The simplicity and modularity of this approach can make a strong impact on fluorescent nanotecnology, specially in the areas of drug delivery and cell taffick analysis, with broad application in the wider field of biomedicine.
Fig. 1 (a) Nanoparticle preparation scheme: sequential enzymatic oxidation of Boc-Try-OH into a reactive product that generates polymers, which can be doped with free aminoacids to form fluorescent nanoparticles. (b) Cryo-TEM and (c) AFM images of the afforded nanoparticles. (d) Visible emission of different nanoparticle formulations irradiated at 365 nm. Adapted from https://doi.org/10.1039/d4nh00400k with permission from the Royal Society of Chemistry.
To find out more, please read:
Full-color peptide-based fluorescent nanomaterials assembled under the control of amino acid doping
Yuhe Shen, Yulin Sun, Yaoyu Liang, Xiaojian Xu, Rongxin Su, Yuefei Wang and Wei Qi
Nanoscale Horiz., 2024, Advance Article
About the blogger
|
|
Understanding the relationship between nanosheets thickness and piezoresistivity in graphene strain sensors
Liquid-phase exfoliation (LPE) is a cheap, scalable and facile way to produce graphene nanosheets. However, what is gained in processability, is then lost in homogeneity of the resulting nanomaterial. This issue is particularly relevant in devices for which inter-nanosheet resistance plays a significant role in their intrinsic performance. For instance, piezoresistive nanosheet-based strain sensors have already been shown to be deeply influenced by network composition and morphology.
In this recent work by Caffrey et al., the influence of nanosheet thickness was investigated and correlated to the piezoresistance of printed graphene sensors. Firstly, the nanosheet suspension was prepared via LPE, different flake sizes were selected through liquid cascade centrifugation (LCC) and their thickness was then estimated via atomic force microscopy (AFM). The team produced different sensors via spray coating a network of graphene nanosheets with thicknesses between 3 and 20 nm (Fig. 1).
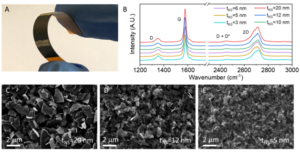
Fig 1. (A) Photograph of a printed sensor. (B) Raman spectra of sprayed graphene films inks of different size-selected nanosheets. (C)–(E) SEM images of networks composed of nanosheets with different size ranges. Reproduced from DOI: 10.1039/D4NH00224E with permission from the Royal Society of Chemistry.
The devices show a clear trend of increasing resistivity and gauge factor with increasing thickness. By using a simple model that correlates the network resistivity with nanosheet thickness, a new model that successfully correlates the gauge factor with thickness was obtained (Fig. 2).
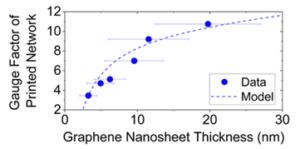
Fig 2. Plot of gauge factor as a function of nanosheet thickness showing both experimental data and the model fitting. Reproduced from DOI: 10.1039/D4NH00224E with permission from the Royal Society of Chemistry.
The authors carefully analyzed the different contributions to the gauge factor and were able to differentiate between the effect of straining of the nanosheets themselves and of the inter-nanosheet junctions. From fitting this model, they observed that, interestingly, strain has a significant influence on nanosheet resistivity, which means that applied strain not only makes the flakes in the network slide on each other, but also induces a detectable deformation on the flakes themselves. Unexpectedly, the calculated nanosheets’ gauge factor was negative. This was attributed to a decrease in nanosheet strain with applied strain, possibly due to the relaxation of built-in strain during network formation or the release of point-to-basal plane contacts.
By applying a theoretical approach to experimental data, the authors were able to interpret and quantify the piezoresistive response in disordered graphene networks produced by LPE. Overall, this study presents a step forward in actively understanding the mechanisms behind the piezoresistive behavior of printed graphene sensors.
To find out more, please read:
Quantifying the effect of nanosheet dimensions on the piezoresistive response of printed graphene nanosheet networks
Eoin Caffrey, Jose M. Munuera, Tian Carey and Jonathan N. Coleman
Nanoscale Horiz., 2024, Advance Article
About the blogger

Sara Domenici is a PhD student at Politecnico di Torino (Turin, Italy) under the supervision of Prof. Teresa Gatti. She was born in Verona (Italy) in 1998. In 2020, she obtained her Bachelor’s degree in Chemistry at the University of Padova (Italy). In 2022, she completed the Double Degree Programme between the University of Padova and the Justus-Liebig University in Giessen (Germany), where she spent 12 months, and obtained a Master’s Degree in Chemistry. Her PhD project is focused on Janus two-dimensional materials for energy conversion, but she also works on hydrogel sensors and dye-sensitized solar cells (DSSCs). |
A universal synthetic method for preparing nanoassemblies of quantum dots and organic molecules
The performance of nanomaterials is intricately linked to their size and combination form. Quantum dots (QDs), for instance, exhibit unique electronic and quantum properties due to their size effect. Moreover, the optical and electronic attributes of QD assemblies can be tailored by adjusting the size of individual QDs and their spatial arrangement within the assembly. Thus, it is imperative to explore suitable and universally applicable methods to prepare adjustable QDs and their assemblies.
In a recent study, Akter et al. devised mesoscopic QD assemblies using a novel bio-catalytic nanoparticle shaping (BNS) approach. Specifically, the authors employed L-lysine as a linker to assemble CdSe/CdS QDs, initially yielding ultra-large QD assemblies. Subsequently, these assemblies were catalytically cleaved by trypsin, resulting in mesoscopic QD assemblies (ms-QD) with a size of 84 nm (Fig. 1A). Relative to single QDs, the redshift and weakened emission observed in the photoluminescence spectrum of ms-QD suggest the presence of internal emission reabsorption processes within the assembly, which holds promise for leveraging energy or electron/hole transfer processes in the production of new optical materials.
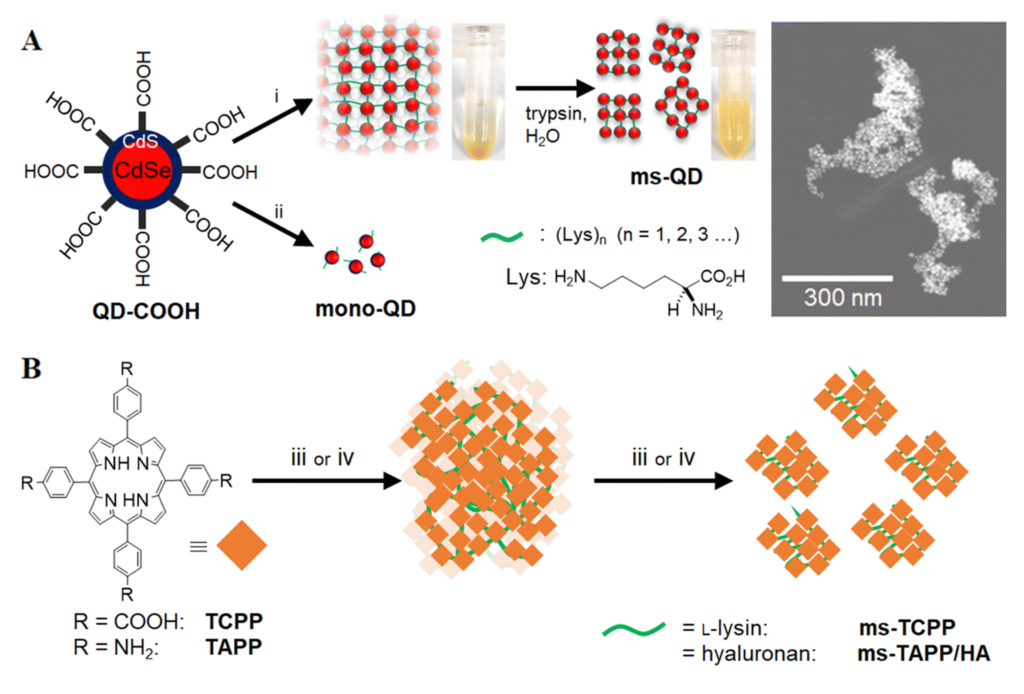
Fig 1. (A) Synthetic pathway of ms-QD by the BNS method and corresponding TEM image, and (B) the synthetic scheme of organic molecule assemblies. Reproduced from DOI: 10.1039/D4NH00134F with permission from the Royal Society of Chemistry.
Additionally, the authors synthesized and investigated nanoassemblies of the organic molecule tetrakis(4-carboxyphenyl)porphyrin (Fig. 1B) using a similar method. They observed significant differences in reactive oxygen generation under light among the various assemblies, indicating the potential to modulate the molecular function of the particle’s core unit by altering the composition of connecting molecules.
In summary, this method demonstrates high versatility and can be employed for preparing assemblies of both QDs and organic molecules. Furthermore, it allows for the replacement of biological enzymes/substrates as needed to generate various nanoassemblies with unique physicochemical properties for further applications.
To find out more, please read:
Bio-catalytic nanoparticle shaping for preparing mesoscopic assemblies of semiconductor quantum dots and organic molecules
Rumana Akter, Nicholas Kirkwood, Samantha Zaman, Bang Lu, Tinci Wang, Satoru Takakusagi, Paul Mulvaney, Vasudevanpillai Biju and Yuta Takano
Nanoscale Horiz., 2024, Advance Article
About the blogger

Chao Wang is a postdoctoral fellow at Oregon State University and a member of the Nanoscale Horizons Community Board. His research focuses on multiple nanomedicines (especially metal-organic frameworks) and their theranostics for cancer, endometriosis and stem cell tracking. |
In vitro nanomaterial testing: unveiling biases through biomolecular corona influence
Currently, nanomaterials (NM) are attracting significant attention in the field of biomedicine. However, once these nanomaterials are utilized for in vivo treatments they interact with the surrounding physiological environment, leading to the adsorption of various biomolecules onto their surfaces, forming a biomolecular corona (BMC) and thereby influencing the performance and behavior of the nanomaterials. Presently, the in vitro studies of NM primarily involve dispersing the nanoparticles in 10% fetal bovine serum (FBS) and then evaluating their toxicity and therapeutic effects. However, this evaluation method is insufficient as it cannot accurately simulate the conditions of human blood. Moreover, this practical issue remains unresolved to date.
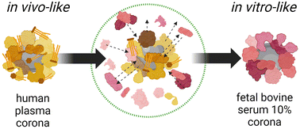
Fig 1. Schematic illustrating the molecular and biological biases arising from the well-known in vitro/in vivo mismatch in nanomedicine due to the biomolecular corona. Reproduced from DOI: 10.1039/D3NH00510K with permission from the Royal Society of Chemistry.
To validate the series of biases existing in established experimental practices and to advance the fields of nanomedicine and nanotoxicology, this study investigated two NM types with vastly different physicochemical properties commonly used in biomedicine. The research compared the molecular and biological biases resulting from the mismatch between NM dispersed in 10% FBS (utilized for in vitro biological assays) and whole human plasma (HP, closer to in vivo administration schemes). Through comparative analysis using proteomics, lipidomics, high-throughput multi-parameter in vitro screening, and single-molecule feature analysis, it was demonstrated that the dynamic changes in BMC composition are material dependent and that cell viability, transport pathways, and autophagic cascades are influenced by the presence or absence of pre-formed BMC corona. These findings underscore the potential limitations of NM in vitro testing in accurately representing real in vivo conditions. Therefore, it is necessary to establish new shared protocols to enhance the accuracy and predictive capability of NM testing.
In summary, this study confirms the biases that may exist when using standard in vitro conditions for NM toxicology assessments, reminding us of the need to establish a comprehensive experimental framework to generate and support new knowledge in the field of biologically relevant nanomaterial interactions. For instance, integrating advanced predictive tools such as artificial intelligence and machine learning will enable nanotoxicology and nanomedicine to progress towards personalized solutions for precision healthcare.
To find out more, please read:
Sources of biases in the in vitro testing of nanomaterials: the role of the biomolecular corona
Valentina Castagnola, Valeria Tomati, Luca Boselli, Clarissa Braccia, Sergio Decherchi, Pier Paolo Pompa, Nicoletta Pedemonte, Fabio Benfenati and Andrea Armirotti
Nanoscale Horiz., 2024, Advance Article
About the blogger

Fangfang Cao is a Research Fellow at National University of Singapore and a member of the Nanoscale Horizons Community Board. Dr Cao’s research focuses on nanocatalytic medicine and microbial therapy. |
Time’s dance with gold: tracking the isomeric fluctuations of Au clusters
Face-centered cubic (fcc) metals, such as Au and Ag, usually adopt a packed crystal structure in bulk. However, the equilibrium structure could differ when only a handful of atoms compose a nanocluster. Theories have predicted that particles less than a few nanometers would favor a decahedral packing with a five-fold symmetry; when even fewer atoms are present, say less than two hundred, a 20-fold icosahedral packing would become the lowest-energy configuration. Such fluctuations of the nuclei/seeds may have played a critical role in defining the shape of colloidal nanoparticles in many wet chemical syntheses.
In recent work, a cross-institutional team led by Richard E. Palmer and Thomas J. A. Slater reported the direct observation of such fluctuations on a nearly second-by-second basis. The team synthesized Au nanoclusters containing 309±15 atoms on an amorphous carbon film through mass-selected magnetron sputtering. Subsequently, aberration-corrected scanning transmission electron microscopy (STEM) was employed to track the atomic structures of Au nanoclusters with a frame rate of 0.4–0.7 per second (Fig. 1). To identify the cluster type in each frame, the team compared them to a collection of simulated images with different cluster structures and tilt angles. The clusters exhibited highly dynamic switching between decahedral, icosahedral, and single-crystalline structures under the electron beam, which is sufficiently strong to overcome the energy barriers between such transitions.
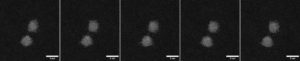
Fig. 1 Au309±15 clusters fluctuating under the electron beam. High-angle annular dark field (HAADF) imaging on an aberration-corrected scanning transmission electron microscope (STEM) resolved the atomic structure of these Au nanoclusters frame by frame. Adapted from the supporting data DOI: 10.5281/zenodo.10522408, CC-BY 4.0.
Notably, the authors showed that the Au309±15 clusters favor the decahedral structure the most, followed by icosahedral and then single-crystalline structures (Fig. 2a). This result is consistent with the probabilities obtained from a snapshot of an ensemble. In theory, the lower-energy structures would have a higher probability of appearance. The ranking of isomeric preferences observed in this study indicates that the cluster size is within a range where the energy ranks in fcc > icosahedral > decahedral (Fig. 2b). Taken together, this work illustrates the possibility of atomic-resolution electron microscopy, when combined with image simulations, to track the isomeric evolution of metal nanoclusters and may shed light on how we understand and regulate nanostructures with atomic precision.
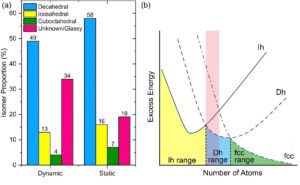
Fig. 1 (a) Histogram of isomer abundances from dynamic movies compared with a static image of a cluster ensemble. Reproduced from DOI: 10.1039/D3NH00291H with permission from the Royal Society of Chemistry. (b) Schematic energy landscape of cluster structures for fcc metals. A red shade indicates the cluster size range in the current study. Ih: icosahedral. Dh: decahedral. Adapted from DOI: 10.1002/anie.202015166 with permission from Wiley-VCH.
To find out more, please read:
Frame-by-frame observations of structure fluctuations in single mass-selected Au clusters using aberration-corrected electron microscopy
Malcolm Dearg, Cesare Roncaglia, Diana Nelli, El Yakout El Koraychy, Riccardo Ferrando, Thomas J. A. Slater, and Richard E. Palmer
Nanoscale Horiz., 2024, 9, 143-147
About the blogger

Jingshan S. Du is a Washington Research Foundation Postdoctoral Fellow at Pacific Northwest National Laboratory and a member of the Nanoscale Horizons Community Board. His research spans crystal formation and transformation pathways, in situ electron microscopy, and hybrid organic/inorganic nanostructures. Du received a Ph.D. in Materials Science and Engineering from Northwestern University in 2021. At Northwestern, he worked on complex nanoparticle systems, correlative electron microscopy of hybrid nanostructures, and nanoscale thermodynamics. Du received a Certificate for Management for Scientists and Engineers from Northwestern’s Kellogg School of Management in 2021 and a B.Sc. in Engineering from Zhejiang University Chu Kochen Honors College in 2015. You can follow him on Twitter @JingshanDu. The views expressed in this article do not necessarily reflect those of the author’s employer or the US government.
|
Injectable hydrogel reinforces cancer immunotherapy
In the last few years, immunotherapy has paved new paths for effective treatment of different cancers. Specifically, immunotherapy stimulates T cells, a type of white blood cell called lymphocytes that help to fight germs and destroy tumours. Immunotherapy can be used as a monotherapy or combined with chemotherapy and surgery. Unfortunately, cancer cells and their microenvironment have many sophisticated defence mechanisms that pose considerable challenges to immunotherapy effectiveness and progress. Current strategies to boost cancer immunotherapy include increasing the infiltration of T cells at the tumour site or blocking immune checkpoint-producing immune evasion.
In this regard, an exciting immunotherapy combination approach has been developed by Guixiang Xu and team based on an injectable hydrogel as a carrier to deliver a drug called linagliptin which is capable of inhibiting dipeptidyl peptidases 4 (DPP4) degradation. This leads to prolonged half-life of CXCL10 chemokines and thus, increases recruitment of T cells in the tumour site. Small molecule immune checkpoint blocker (BMS-202) particles were also loaded onto the developed drug carrier to block the programmed cell death-ligand (PD-L1), avoiding immune evasion. The team demonstrated that the application of hydrogel construct (S@LB) suppresses chemokine CXCL10 degradation, increasing T-cell infiltration, while BMS-202 particles inactivate PD-L1 checkpoint in vivo.
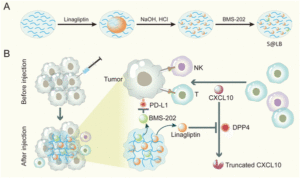
Fig. 1 Preparation and mechanism scheme of S@LB. (A) The preparation process of the S@LB solution. (B) Schematic illustration of an injectable hydrogel to reinforce cancer immunotherapy by promoting infiltration of T cells and regulating immune evasion. Reproduced from DOI: 10.1039/D3NH00401E with permission from the Royal Society of Chemistry.
The team tested the in vivo anti-tumour ability, immune response, and lung anti-metastatic effect of the S@LB in combination with chemotactic CXCL10 (S@LB + CXCL10). Their recent report shows that after 18 days of tumour removal, an immune memory effect was detected for the group treated with S@LB + CXCL10.
Overall, this study shows how nano-based hydrogel immunotherapy can be used as an innovative “weapon” against primary and distant tumours, along with efficient inhibition of lung metastasis, indicating tremendous potential for developing transformative clinical applications.
To find out more, please read:
Hydrogel-mediated tumor T cell infiltration and immune evasion to reinforce cancer immunotherapy
Guixiang Xu, Kai Liu, Xiangwu Chen, Yang Lin, Cancan Yu, Xinxin Nie, Wenxiu He, Nathan Karinc and Yuxia Luan
Nanoscale Horiz., 2024, Advance Article
About the blogger

Susel Del Sol Fernández is a Marie Skłodowska-Curie Postdoctoral fellow at Aragon Nanoscience and Materials Institute (INMA-CSIC), Spain and a member of the Nanoscale Horizons Community Board. Dr Del Sol’s research focuses on designing smart functionalized magnetic nanoparticles for biomedical applications, including magnetic-optical hyperthermia treatment and magnetogenetics. You can follow her on X @SuselDelSol
|
Measuring carrier density and mobility in single-walled carbon nanotubes via nuclear magnetic resonance
The rapidly expanding energy and computing sectors are driving demand for high-performance semiconductor materials. Organic Semiconductors (OSCs) have emerged as attractive candidates for opto-electronic devices, thanks to their high carrier mobility, stability and tunability. However, accurate and independent quantification of charge carrier density and mobility has been an ongoing challenge in the OSC community. Working to overcome this challenge, recent research by Hermosilla-Palacios et al. presents a novel method for determining charge carrier characteristics in semiconducting single-walled carbon nanotubes (s-SWCNTs), a subtype of OSCs.
In this study, a nuclear magnetic resonance (NMR)-based approach is proposed to directly quantify charge carrier density and indirectly quantify carrier mobility (Fig. 1). The study puts forward a combined method utilizing 19F NMR and optical absorption measurements on s-SWCNTs in the presence of F-containing molecular dopants. The researchers demonstrated that changes in carrier density affect charge delocalization, resulting in a carrier density-dependent mobility, in contrary to that expected for mobility limited by ionized impurity scattering. This combined approach simplifies the measurement of carrier density in doped s-SWCNTs, constituting a valuable tool to the OSC community.
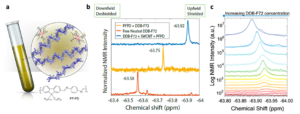
Fig. 1 (a) Cartoon showing the NMR tube sample composition: polymer dispersed s-SWCNT, excess polymer (in blue) and DDB-F72 molecules associated with a hole on the doped s-SWCNTs. Repeating unit of the polymer PF-PD is also presented for clarity. (b) Spectra corresponding to 19F NMR for neutral DDB-F72 dopant in d8-toluene (6 mM, bottom), PF-PD polymer used to disperse s-SWCNTs with added DDB-F72 (6 mM, middle), and dispersed s-SWCNTs with added DDB-F72 (6 mM, top). Numbers show specific chemical shift. (c) Spectra corresponding to 19F NMR for doping series of s-SWCNT. Spectra are arbitrarily displaced along the y axis to show the different doping steps clearly. Lower dopant concentration (red) to higher dopant concentrations (blue). Reproduced from DOI: 10.1039/D3NH00480E with permission from the Royal Society of Chemistry.
While this study presents significant strides in measuring carrier density in s-SWCNTs, whether it can be effectively applied to a wide range of OSCs beyond s-SWCNTs remains to be seen. It should also be noted that the downfield shift observed with increasing dopant concentration may be complicated by factors other than charge delocalization of the hole distribution, such as dopant binding dynamics. The mechanistic origin of the chemical shift changes in the presence of dopants with NMR -active nuclei may refine our understanding of the local micro-environment around the redox-doped s-SWCNTs, prompting further investigations in this area.
In summary, this study develops an NMR-based method to quantify charge carrier density in s-SWCNTs and illustrates that the hole mobility in doped s-SWCNT networks increases with growing carrier density. The ability to tune, quantify, and optimize carrier density opens new avenues for applications such as photovoltaics, sensors, light-emitting diodes, field-effect transistors, and thermoelectric devices. The method’s potential applicability to various p-conjugated semiconductors using suitable NMR-active dopants makes it a versatile tool for the field. As the scientific community embraces this innovative approach, it heralds a new chapter in the design and development of high-performance semiconductor materials.
To find out more, please read:
Carrier density and delocalization signatures in doped carbon nanotubes from quantitative magnetic resonance
M. Alejandra Hermosilla-Palacios, Marissa Martinez, Evan A. Doud, Tobias Hertel, Alexander M. Spokoyny, Sofie Cambré, Wim Wenseleers, Yong-Hyun Kim, Andrew J. Ferguson and Jeffrey L. Blackburn
Nanoscale Horiz., 2024, Advance Article
About the blogger

Albert Liu is an Assistant Professor at the University of Michigan, and a member of the Nanoscale Horizons Community Board. Prof. Liu’s research group studies the effects of micro-confinement in nano-structured low dimensional materials, to address challenges in sustainability, robotics, and healthcare. You can follow Albert on Twitter @Albert_T_Liu |
NIR-triggered multifunctional CuS-embedded nanogels for advanced chronic wounds therapy
Chronic wounds are considered a major healthcare problem all over the world. Long-term infections and/or suppressed immune responses may cause chronic wounds with slower healing, resulting in increased mortality. Although antibiotics, skin disinfectants, and hydrogels are currently being used to combat microbial pathogenesis, they still have some significant limitations when used in clinical wound healing. Therefore, researchers have been exploring alternative approaches, such as combining antimicrobial, antioxidant, and anti-inflammatory agents for advanced chronic wound therapy.
Recently, nanomaterial-based antimicrobials have gained popularity thanks to catalytic and near-infrared (NIR) irradiation treatments, which induce controlled oxidative stress (photodynamic and catalytic therapies) and hyperthermia (photothermal therapies) to eradicate bacteria. However, little research into nanomaterial-based antimicrobial activity against biofilms and chronic wound healing in vivo has previously been reported.
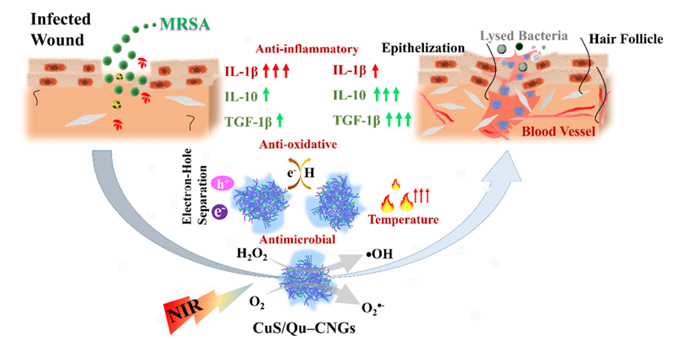
Fig. 1 An overview of the properties of CuS/Qu–CNGs and their role in wound healing. Reproduced from DOI: 10.1039/D3NH00275F with permission from the Royal Society of Chemistry.
In this regard, an NIR-triggered multifunctional quercetin carbonized nanogel embedded with copper sulfide nanoclusters (CuS/Qu-CNG) was reported by Nain et al. for advanced therapy of chronic wounds. Polymerization and mild carbonation procedures were used to prepare quercetin carbonized nanogels (Qu-CNGs), which were subsequently used as templates to grow CuS in situ forming CuS/Qu-CNGs. The resulting CuS/Qu–CNGs are photoreactive and contain antioxidant and catalytic properties (oxidase- and peroxidase-like activities). As a result of their photo-responsive properties, CuS/Qu-CNGs significantly amplified their antimicrobial activity when exposed to NIR-II light. A CuS/Qu–CNGs MIC90 value of 6–9 mg mL-1 is ~125-fold lower than Qu or Qu–CNGs under NIR-II irradiation and was further improved by ~30-fold (ca. 0.2 mg mL-1) in the presence of H2O2. Besides, CuS/Qu-CNGs demonstrated exceptional penetration ability, eliminating MRSA biofilms caused by diabetic wounds in diabetic mice. By suppressing pro-inflammatory cytokines (IL-1β) and boosting anti-inflammatory cytokines (IL-10 and TGF-β1), CuS/Qu-CNGs significantly accelerated wound healing by promoting angiogenesis, epithelialization and collagen synthesis. Finally, CuS/Qu–CNGs showed superior in vivo efficacy in treating bacterial infections and enhancing wound healing in diabetic mice.
In summary, a “Three in One” multifunctional CuS/Qu-CNGs with excellent antimicrobial/antioxidative/anti-inflammatory properties demonstrate great potential in treating bacterial infections and promoting chronic wound healing. This work is expected to provide new solutions for wound treatment complicated by microbial pathogenesis.
To find out more, please read:
NIR-activated quercetin-based nanogels embedded with CuS nanoclusters for the treatment of drug-resistant biofilms and accelerated chronic wound healing
Amit Nain, Yu-Ting Tseng, Akash Gupta, Yu-Feng Lin, Sangili Arumugam, Yu-Fen Huang, Chih-Ching Huang and Huan-Tsung Chang
Nanoscale Horiz., 2023, 8, 1652-1664
About the blogger

Jiangjiexing Wu is an Associate Professor at Tianjin University and a member of the Nanoscale Horizons Community Board. Dr Wu’s research focuses on the rational design and synthesis of functional nanomaterials (such as nanozymes) for analytical, biomedical, and environmental applications. |
Defect engineering enhances CO2 reducing photocatalysts
Recycling CO2 molecules through photocatalysis represents an innovative technology to mitigate the emission of CO2 gas. The deliberate introduction of defects into a photocatalyst plays a crucial role in optimizing photocatalytic performance, since the defects regulate the electronic structure, fine-tune selectivity by enhancing catalytic activity, and reduce the activation barrier of the catalyst.
A team of researchers based in Australia have recently developed a photocatalytic system for CO production by CO2 reduction. In this study, defect-rich g-C3N4 serves as a semiconducting substrate, and it was further loaded with Ag nanoparticles (NPs) to act as a plasmonic source, resulting in the creation of the g-C3N4-Ag photocatalyst. The defects within the g-C3N4 were known as the active sites that enhance the efficiency of photocatalytic CO2 conversion. In addition, these defects are strategically positioned alongside the loaded Ag NPs, which improves the effectiveness of injected hot electrons from the Ag NPs, thereby synergistically enhancing the activity of photocatalytic CO2 reduction.
In the experiments involving defect-rich g-C3N4, different photodeposition times, including 10 minutes, 1 hour, 3 hours, and 5 hours, were performed to load Ag particles, denoted the as-preared photocatalyst as g-C3N4-Ag 10m, 1h, 3h, and 5h, respectively. These variants were evaluated for their photocatalytic performance in CO production via CO2 reduction (Fig. 1a). The optimal performance was achieved with g-C3N4-Ag 1h, and the production of CO was confirmed through isotopic experiments (Fig. 1b). The g-C3N4-Ag photocatalysts were characterized using scanning transmission electron microscopy, as typical image is presented in Fig. 1c and the corresponding elemental mapping were presented in Fig. 1d to 1g.
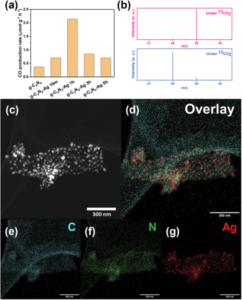 Fig 1. (a) CO production rate based on various g-C3N4 based photocatalysts. (b) Isotope labelling experiments tested under 13CO2 and 12CO2, and the mass spectrometry signals at m/z = 28 and m/z = 29 are 13CO and 12CO, respectively. (c) STEM dark field image and (d)–(g) elemental mapping of g-C3N4-Ag 1h catalyst. Scale bar: 300 nm. Reproduced from DOI: 10.1039/D3NH00348E with permission from the Royal Society of Chemistry. |
To elucidate the mechanism behind the defect engineering scenario, Ag NPs were initially loaded onto g-C3N4 via photodeposition. Due to the electron-rich environment of the point defects on g-C3N4, Ag+ ions selectively grow on these defect sites. The resulting g-C3N4-Ag composite was subsequently annealed. During this process, the new defects formed on the g-C3N4 substrate owing to the strain induced by the differing thermal expansion rates between the Ag and g-C3N4. These new defects were found to be located around the Ag NPs, representing a significant change in the pristine g-C3N4 following the introduction of Ag.
Furthermore, density functional theory (DFT) calculations were conducted to gain a deeper understanding of how the defects in g-C3N4 improve performance. Three models of photocatalysts were considered, including pristine g-C3N4, g-C3N4 with N vacancies, and N vacancies in g-C3N4 with O sites on the surface. In the models of pristine g-C3N4 and g-C3N4 with N vacancies, the formation of *COOH intermediates was identified as the rate-limiting step (RDS), and moreover, N vacancies in g-C3N4 were found to enhance the activity in this conversion (Fig 2a). For N vacancies in g-C3N4 with additional surface O sites (Fig. 2b), the initial reaction step favored the formation of *COOH intermediates from a thermodynamic perspective. Subsequently, the reduction of *COOH intermediates to *CO species occurred by reacting with protons, releasing H2O molecules. In the case of O-enriched g-C3N4, this conversion became the RDS. DFT calculations indicated that the ΔG values for *COOH and *H to form *CO and H2O at the C defect active sites were 0.96 eV, which determined the reaction rate (Fig. 2c). These results provide insight into the reasons behind the improved performance in CO production through CO2 reduction.
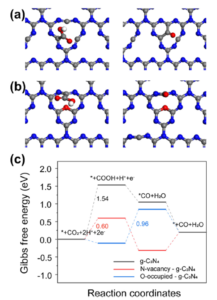 Fig 2. Optimized configurations of reaction intermediates *COOH and *CO on the C atom and N vacancy active sites of (a) g-C3N4 with N vacancy and (b) N vacancies in g-C3N4 with O sites on the surface. (Red ball is oxygen atom, white ball is hydrogen atom, gray is carbon atom, and blue is nitrogen atom) (c) Gibbs free energy diagrams for photocatalytic CO2 reduction to CO on g-C3N4, g-C3N4 with N vacancy and O-occupied g-C3N4. Reproduced from DOI: 10.1039/D3NH00348E with permission from the Royal Society of Chemistry. |
In summary, the deliberate introduction of active defects into g-C3N4 photocatalysts, strategically positioned near the plasmon centers of Ag NPs, optimizes the utilization efficiency of plasmonic hot electrons, resulting in an enhanced efficiency for CO2 photoreduction. Importantly, this strategy has the potential for extension to various systems based on polymers, hard materials, and hybrid materials, offering promising applications that harness the functionalities of defects in a wide range of fields.
To find out more, please read the full article:
Defect engineering enhances plasmonic-hot electrons exploitation for CO2 reduction over polymeric catalysts
Hang Yin, Zhehao Sun, Kaili Liu, Ary Anggara Wibowo, Julien Langley, Chao Zhang, Sandra E. Saji, Felipe Kremer, Dmitri Golberg, Hieu T. Nguyen, Nicholas Cox and Zongyou Yin
Nanoscale Horiz., 2023, Advance Article
About the blogger

Yuanxing Fang is a Professor at Fuzhou University, and a member of the Nanoscale Horizons Community Board. Prof. Fang’s research lab focuses on the synthesis of metal-free semiconductors for photoelectrochemical systems for energy and environmental applications, including water splitting, hydrogen peroxide synthesis, organic transformations and others. |