These HOT articles were recommended by our referees and are free to access for 4 weeks*
Hepatic organoids for microfluidic drug screening
Sam H. Au, M. Dean Chamberlain, Shruthi Mahesh, Michael V. Sefton and Aaron R. Wheeler
Lab Chip, 2014, Advance Article
DOI: 10.1039/C4LC00531G, Paper
Delayed voltammetric with respect to amperometric electrochemical detection of concentration changes in microchannels
Raphaël Trouillon and Martin A. M. Gijs
Lab Chip, 2014,14, 2929-2940
DOI: 10.1039/C4LC00493K, Paper
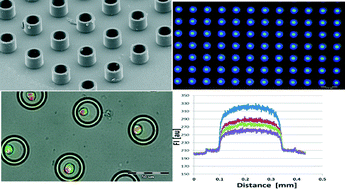
A droplet-based heterogeneous immunoassay for screening single cells secreting antigen-specific antibodies
Samin Akbari and Tohid Pirbodaghi
Lab Chip, 2014, Advance Article
DOI: 10.1039/C4LC00082J, Communication
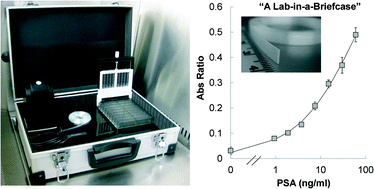
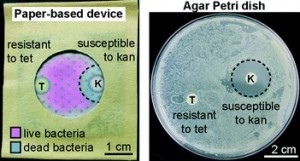
A lab-in-a-briefcase for rapid prostate specific antigen (PSA) screening from whole blood
Ana I. Barbosa, Ana P. Castanheira, Alexander D. Edwards and Nuno M. Reis
Lab Chip, 2014,14, 2918-2928
DOI: 10.1039/C4LC00464G, Paper
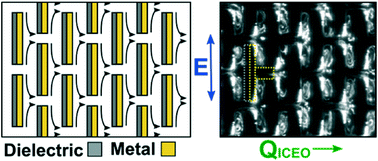
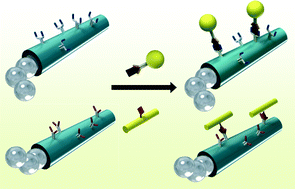
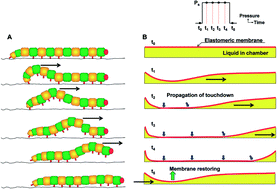
Induced charge electroosmosis micropumps using arrays of Janus micropillars
Joel S. Paustian, Andrew J. Pascall, Neil M. Wilson and Todd M. Squires
Lab Chip, 2014, Advance Article
DOI: 10.1039/C4LC00141A, Paper
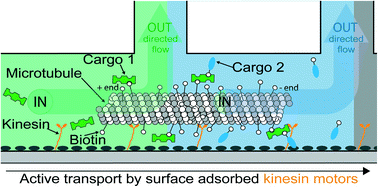
Nanoshuttles propelled by motor proteins sequentially assemble molecular cargo in a microfluidic device
Dirk Steuerwald, Susanna M. Früh, Rudolf Griss, Robert D. Lovchik and Viola Vogel
Lab Chip, 2014, Advance Article
DOI: 10.1039/C4LC00385C, Paper
Femtosecond laser 3D micromachining: a powerful tool for the fabrication of microfluidic, optofluidic, and electrofluidic devices based on glass
Koji Sugioka, Jian Xu, Dong Wu, Yasutaka Hanada, Zhongke Wang, Ya Cheng and Katsumi Midorikawa
Lab Chip, 2014, Advance Article
DOI: 10.1039/C4LC00548A, Critical Review
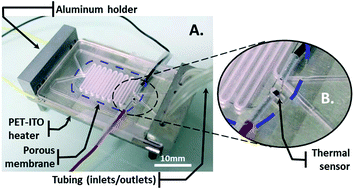
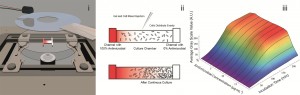
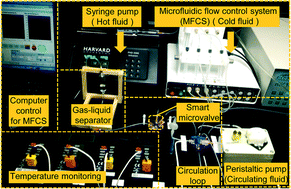
Continuous microcarrier-based cell culture in a benchtop microfluidic bioreactor
F. Abeille, F. Mittler, P. Obeid, M. Huet, F. Kermarrec, M. E. Dolega, F. Navarro, P. Pouteau, B. Icard, X. Gidrol, V. Agache and N. Picollet-D’hahan
Lab Chip, 2014, Advance Article
DOI: 10.1039/C4LC00570H, Paper
Multiplexed immunoassay based on micromotors and microscale tags
D. Vilela, J. Orozco, G. Cheng, S. Sattayasamitsathit, M. Galarnyk, C. Kan, J. Wang and A. Escarpa
Lab Chip, 2014, Advance Article
DOI: 10.1039/C4LC00596A, Paper
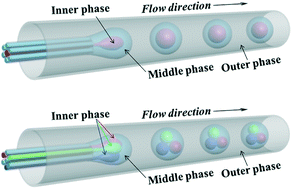
Double emulsions from a capillary array injection microfluidic device
Luoran Shang, Yao Cheng, Jie Wang, Haibo Ding, Fei Rong, Yuanjin Zhao and Zhongze Gu
Lab Chip, 2014, Advance Article
DOI: 10.1039/C4LC00698D, Communication
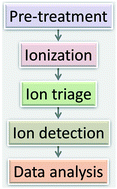
SU-8 as a material for lab-on-a-chip-based mass spectrometry
Steve Arscott
Lab Chip, 2014, Advance Article
DOI: 10.1039/C4LC00617H, Tutorial Review
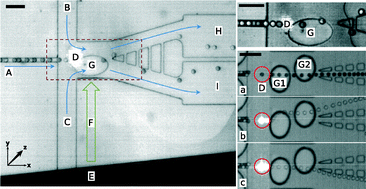
Sorting drops and cells with acoustics: acoustic microfluidic fluorescence-activated cell sorter
Lothar Schmid, David A. Weitz and Thomas Franke
Lab Chip, 2014, Advance Article
DOI: 10.1039/C4LC00588K, Paper
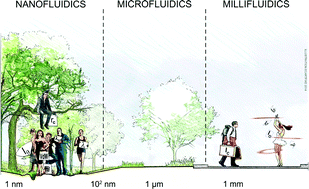
Physics and technological aspects of nanofluidics
Lyderic Bocquet and Patrick Tabeling
Lab Chip, 2014, Advance Article
DOI: 10.1039/C4LC00325J, Frontier
*Free access to individuals is provided through an RSC Publishing personal account. It’s quick, easy and more importantly – free – to register!