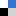Virtually all environmental surfaces have microbial biofilms, which are an essential component of natural systems. Nanoparticles are abundant in nature, but how much do we know about nanoparticle-biofilm interactions?
Kaoru Ikuma and colleagues from the University of Massachusetts Amherst have completed a study highlighting the importance of assessing small-scale biofilm surface characteristics in future nanoparticle-biofilm interaction studies. They hypothesized that the contribution of electrostatic forces in relation to other forces would be dominant in governing the deposition of bare nanoparticles onto polysaccharide-coated surfaces. The also expected biofilm surface charge to impact nanoparticle deposition.
Polysaccharides are a major extracellular component of biofilms. They are ubiquitous in the environment and occur in pure forms as well as in complexes. As polysaccharides are extracellular they are an initial point of contact for nanoparticles and play an important role in early nanoparticle-biofilm interactions. Typical characterization of biofilms often treat all polysaccharides as one entity. This study suggests that the small-scale chemical and electrochemical identities of the polysaccharides present in the biofilms may play an important role in the initial surface attachment of nanoparticles; therefore effecting their deposition.
The significance of polysaccharide coatings on the deposition of nanoparticles was examined using in-depth characterization of surface properties. It would appear that surface charge density and distribution of the biofilms both contribute to different nanoparticle deposition behaviours.
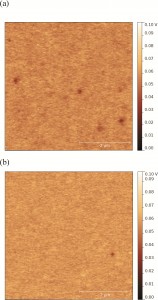
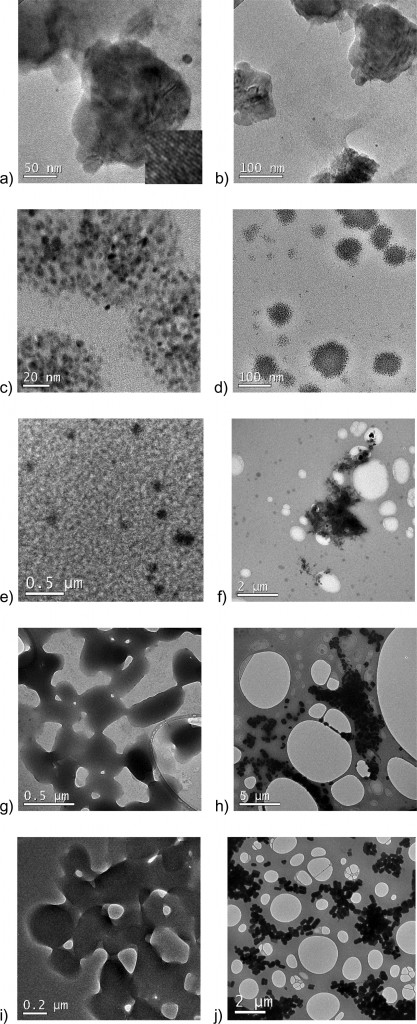
Kelvin probe force microscopy was used as a probe for spatial variations of surface potential across two different polysaccharides (alginate and dextran sulphate). Patches of lower surface potential are observed as the areas of darker colour on the images below.
The results showed that even though the interactions between nanoparticles and surfaces coated with pure polysaccharides may be governed by electrostatic forces, these interactions can be altered by microscale and nanoscale differences in surface charge. Therefore, instead of treating polysaccharides as one entity, spatial characterization of biofilm surface properties is necessary to improve our understanding of nanoparticle-biofilm interactions.
To find out more, download your free* copy by following the link below.
Deposition of nanoparticles onto polysaccharide-coated surfaces: Implications for nanoparticle-biofilm interactions. Kaoru Ikuma, Andrew Madden, Alan Decho and Boris L. T. Lau 10.1039/C3EN00075C
*Access is free through a registered RSC account – click here to register