Typically when people drink water they expect it to be clear, however the effluent discharge by many industries contains a considerable amount of dye. A very small amount of dye contaminating water can be highly visible and is undesirable for several reasons:
- Colour influences negative perception of water quality.
- Colour interferes with light penetration, reducing photosynthesis in aquatic plants and therefore destroying aquatic ecosystems.
- Dyes can be toxic, carcinogenic and mutagenic, which is a serious hazard to aquatic organisms as well as human health.
- Dyes are difficult to remove as most dyes are resistant to biological degradation.
Layered double hydroxides (LHZS) have been an attractive candidate for adsorbents to selectively remove pollutants due to their large surface area, ease of preparation, exchangeable interlayer anions, compositional flexibility and low cost. However Shiyao Zhu and colleagues at Jilin University have prepared CuZn hydroxyl double salts (CuZn-HDS) and tested them as an adsorbent for methyl orange (MO) removal. The adsorption performance of CuZn-HDS was much better than the adsorption performance of LHZS, proving CuZn-HDS to be a promising adsorbent for the removal of dye from wastewater.
Three CuZn-HDS samples were prepared using different water ratios and their adsorption capacities and surface areas were investigated and compared to the adsorption capacities of LHZS.
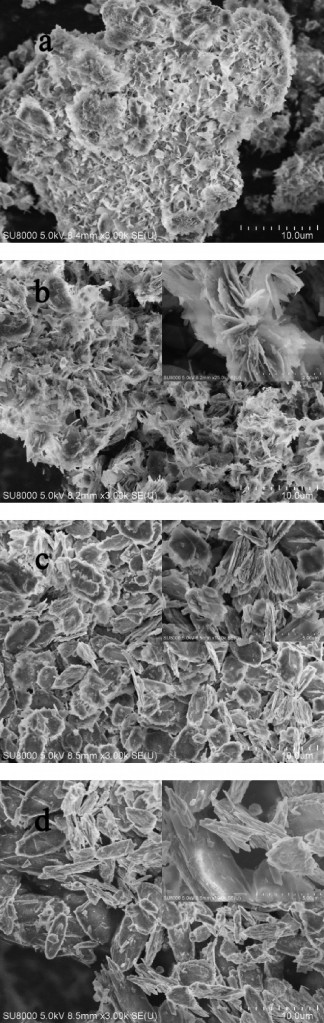
This picture shows the scanning electron microscope images of LHZS (a) and each CuZn-HDS sample after MO adsorption. CuZn-1 (b) had the highest surface area and the highest adsorption capacity for MO due to its multivalve flower-like structure with stacked nanoplatelets.
Due to the increasing environmental pollution from dye wastewater emissions, an important role for these nanomaterials is as potential adsorbents for the removal of pollutants from wastewater.
To find out more, download the full article for free* by clicking the link below.
High adsorption capacity for dye removal by CuZn hydroxyl double salts
Shiyao Zhu, Shihui Jiao, Ziwei Liu, Guangsheng Pang and Shouhua Feng
DOI: 10.1039/C3EN00078H
*Access is free through a registered RSC account – click here to register