Groundwater contamination is becoming a bigger problem than in the past as a result of ever increasing industries and agricultural practices. Furthermore, a significant percentage of drinking water supply relies on clean ground water such that its efficient and effective remediation is a timely need. Specifically chlorinated solvents and nitroaromatic compounds are oxidized organics, which are frequently found as persistent contaminants in groundwater. As a result of their stability in oxic environments these contaminants can act as transporters to a variety of other pollutants between soil and ground waters phases in addition to endangering humans and wildlife.
The oxidized functional groups in these contaminants can be reduced by Fe(II) associated with iron minerals thus providing means for their degradation that can be incorporated into engineered remediation schemes. Ferrihydrite, goethite, magnetite, hematite and lepidocrocite are examples of some ubiquitous iron containing minerals. In these degradation reactions the number of reactive sites on the minerals are directly related to the specific surface area and thereforethe nanoparticles of these minerals, which inherently has large surface areas hold the greatest potential towards degrading these contaminants. Nevertheless, nanoparticles are highly susceptible to aggregation, which can significantly hinder the efficiency of the reduction process. Therefore, Amanda M. Stemig and co-workers from the University of Minnesota, have conducted an extensive investigation using 4-Chloronitrobenzene (4-ClNB) as a model compound to elucidate the link between the aggregation state of iron oxide nanoparticles and their reactivity.
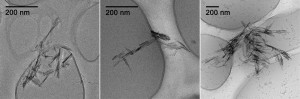
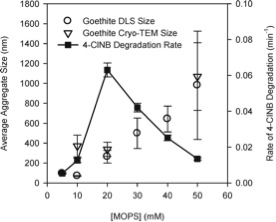 The study was conducted by using well-characterized goethite nanoparticles as the iron containing minerals. The results revealed that the size of the goethite nanoparticles are significantly reduced upon the adsorption of transition metals. This is, of course no surprise as the adsorption of transition metals introduces additional surface charge. Furthermore, a comparison between the pseudo first order rate constants of 4-ClNB degradation in a variety of buffers indicated that the buffer type affected the reaction kinetics by controlling the aggregation state and thereby changing the available surface area. It was clearly demonstrated that zwitterionic buffers with spatial charge separations are better at preventing aggregation, giving better degradation rates. In addition buffer concentration also affected the degradation kinetics as higher buffer concentrations resulted in more densely packed aggregates with lowered surface area.
The study was conducted by using well-characterized goethite nanoparticles as the iron containing minerals. The results revealed that the size of the goethite nanoparticles are significantly reduced upon the adsorption of transition metals. This is, of course no surprise as the adsorption of transition metals introduces additional surface charge. Furthermore, a comparison between the pseudo first order rate constants of 4-ClNB degradation in a variety of buffers indicated that the buffer type affected the reaction kinetics by controlling the aggregation state and thereby changing the available surface area. It was clearly demonstrated that zwitterionic buffers with spatial charge separations are better at preventing aggregation, giving better degradation rates. In addition buffer concentration also affected the degradation kinetics as higher buffer concentrations resulted in more densely packed aggregates with lowered surface area.
To access the full article, download a copy for free* by clicking the link below.
Amanda M. Stemig, Tram Anh Do, Virany M. Yuwono, William A. Arnold and R. Lee Penn
DOI: 10.1039/c3en00063j
*Access is free through a registered RSC account – click here to register