l
The exciting chemistry of metal-organic frameworks (MOF’s) attracts interest from a range of communities within the chemical sciences. Recently the Journal of Materials Chemistry published a themed issue ‘Integrating functionality into metal–organic frameworks’ and Dalton Transactions ‘Coordination chemistry in the solid state’.
You can see the full issues by clicking on the links above, alternatively you can browse the highlights below, which are free to download until the 27th July.
Metal–organic frameworks as scaffolds for the encapsulation of active species: state of the art and future perspectives
Jana Juan-Alcañiz, Jorge Gascon and Freek Kapteijn
J. Mater. Chem., 2012,22, 10102-10118
ll
l
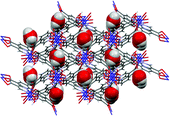
Supramolecular isomers of metal–organic frameworks: the role of a new mixed donor imidazolate-carboxylate tetradentate ligand
Victoria J. Richards, Stephen P. Argent, Adam Kewley, Alexander J. Blake, William Lewis and Neil R. Champness
Dalton Trans., 2012, 41, 4020-4026
Effect of the organic functionalization of flexible MOFs on the adsorption of CO2
Thomas Devic, Fabrice Salles, Sandrine Bourrelly, Béatrice Moulin, Guillaume Maurin, Patricia Horcajada, Christian Serre, Alexandre Vimont, Jean-Claude Lavalley, Hervé Leclerc, Guillaume Clet, Marco Daturi, Phillip L. Llewellyn, Yaroslav Filinchuk and Gérard Férey
J. Mater. Chem., 2012, 22, 10266-10273
Highly oriented surface-growth and covalent dye labeling of mesoporous metal–organic frameworks
Florian M. Hinterholzinger, Stefan Wuttke, Pascal Roy, Thomas Preuße, Andreas Schaate, Peter Behrens, Adelheid Godt and Thomas Bein
Dalton Trans., 2012, 41, 3899-3901
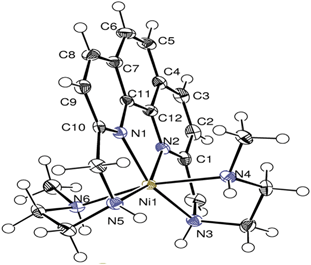
Structural flexibility and intrinsic dynamics in the M2(2,6-ndc)2(dabco) (M = Ni, Cu, Co, Zn) metal–organic frameworks
Nicole Klein, Herbert C. Hoffmann, Amandine Cadiau, Juergen Getzschmann, Martin R. Lohe, Silvia Paasch, Thomas Heydenreich, Karim Adil, Irena Senkovska, Eike Brunner and Stefan Kaskel
J. Mater. Chem., 2012, 22, 10303-1031
 Hydrogen adsorption in the metal–organic frameworks Fe2(dobdc) and Fe2(O2)(dobdc)
Hydrogen adsorption in the metal–organic frameworks Fe2(dobdc) and Fe2(O2)(dobdc)
Wendy L. Queen, Eric D. Bloch, Craig M. Brown, Matthew R. Hudson, Jarad A. Mason, Leslie J. Murray, Anibal Javier Ramirez-Cuesta, Vanessa K. Peterson and Jeffrey R. Long
Dalton Trans., 2012, 41, 4180-4187
You might also be interested in the recent CrystEngComm Highlight
Coordination polymers, metal–organic frameworks and the need for terminology guidelines
Stuart R. Batten, Neil R. Champness, Xiao-Ming Chen, Javier Garcia-Martinez, Susumu Kitagawa, Lars Öhrström, Michael O’Keeffe, Myunghyun Paik Suh and Jan Reedijk
CrystEngComm, 2012, 14, 3001-3004
Comments Off on Highlights from themed issues covering metal–organic frameworks
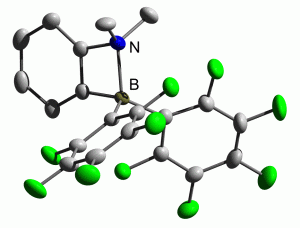 A frustrated Lewis pair, in this case, is a molecule that contains a Lewis acid group and a Lewis base group kept apart due to sterics. Such compounds are, perhaps unsurprisingly, very reactive. The most important practical application of FLPs is most likely to be the catalysed hydrogenation of polar double bonds under ambient conditions.
A frustrated Lewis pair, in this case, is a molecule that contains a Lewis acid group and a Lewis base group kept apart due to sterics. Such compounds are, perhaps unsurprisingly, very reactive. The most important practical application of FLPs is most likely to be the catalysed hydrogenation of polar double bonds under ambient conditions.











 Hydrogen adsorption in the metal–organic frameworks Fe2(dobdc) and Fe2(O2)(dobdc)
Hydrogen adsorption in the metal–organic frameworks Fe2(dobdc) and Fe2(O2)(dobdc)






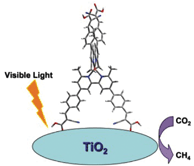 Photochemical reduction of greenhouse gas, carbon dioxide into higher energy content fuels is attractive because it utilises a renewable energy source – the sun. Most catalysts that are already used for this process (TiO2 and metallic clusters based on Cu, Pt and Rh) need ultraviolet light for excitation and have a low conversion efficiency, prohibiting wide scale industrial application.
Photochemical reduction of greenhouse gas, carbon dioxide into higher energy content fuels is attractive because it utilises a renewable energy source – the sun. Most catalysts that are already used for this process (TiO2 and metallic clusters based on Cu, Pt and Rh) need ultraviolet light for excitation and have a low conversion efficiency, prohibiting wide scale industrial application.