Fabrication of high performance, multi-layered phosphorescent organic light-emitting diodes (PhOLEDs) is often done by vacuum deposition of small organic molecules which can be a lengthy and costly process. Solution processing (layers can either be coated or printed from solution) is a more viable technique in terms of cost. However when it is used to make multilayered devices the lower layer can be affected by the solvent from upper layers due to inter-miscibility. It would be better to have electron and hole transporter materials together in a single layered device.
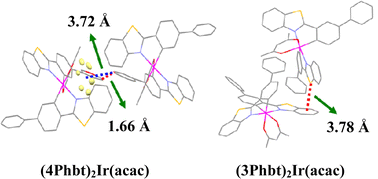
Yan Huang, Zhiyun Lu and colleagues have reported two novel orange iridium(III) complexes. The complexes were synthesized by modifying the phenyl moiety of the parent complex, (bt)2Ir(acac) (bt = phenylbenzothiazole, acac = acetylacetonate). The new complexes have good thermostability and film amorphism meaning that they were able to be tested in a solution-processed single layer PhOLED. Both complexes demonstrated higher luminescence and efficiency than the parent complex. The improved characteristics of the two new Ir(III) complexes mean that they are promising candidates for use in low cost fabrication of PhOLEDs for large area displays.
To find out more, read the full Dalton Transactions article:
Iridium(III) complexes with enhanced film amorphism as guests for efficient orange solution-processed single-layer PhOLEDs with low efficiency roll-off
Jun Dai, Kaifeng Zhou, Ming Li, Huiqin Sun, Yunqing Chen, Shijian Su, Xuemei Pu, Yan Huang and Zhiyun Lu
Dalton Transactions, DOI: 10.1039/C3DT50834J











![Recyclable calix[4]arene–lanthanoid luminescent hybrid materials with color-tuning and color-switching properties](https://blogs.rsc.org/dt/files/2013/04/GA1.gif)