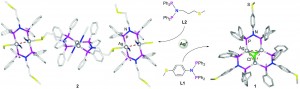
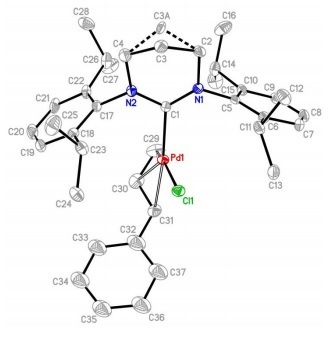
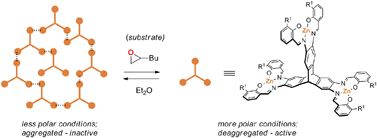
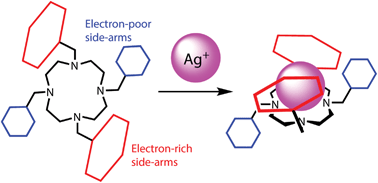
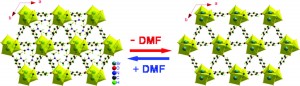
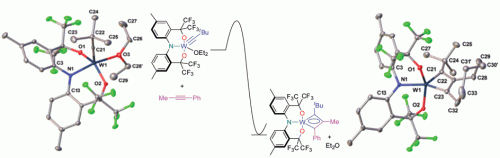
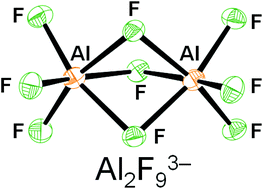
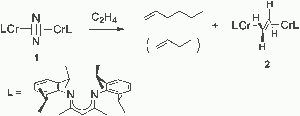Influence of a thioether function in short-bite diphosphine ligands on the nature of their silver complexes: structure of a trinuclear complex and of a coordination polymer
Vitor Rosa, Christophe Fliedel, Alessio Ghisolfi, Roberto Pattacini, Teresa Avilés and Pierre Braunstein
Dalton Trans., 2013, DOI: 10.1039/C3DT50555C
Free to access until 24th May
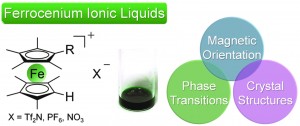
Organometallic ionic liquids from alkyloctamethylferrocenium cations: thermal properties, crystal structures, and magnetic properties
Yusuke Funasako, Takashi Inagaki, Tomoyuki Mochida, Toshihiro Sakurai, Hitoshi Ohta, Ko Furukawa and Toshikazu Nakamura
Dalton Trans., 2013, DOI: 10.1039/C3DT00084B
Free to access until 24th May
Europium doped In(Zn)P/ZnS colloidal quantum dots
Ung Thi Dieu Thuy, Axel Maurice, Nguyen Quang Liem and Peter Reiss
Dalton Trans., 2013, DOI: 10.1039/C3DT50526J
Free to access until 24th May
A naphthalene–thiophene hybrid molecule as a fluorescent AND logic gate with Zn2+ and OAc− ions as inputs: cell imaging and computational studies
Debasis Karak, Sudipta Das, Sisir Lohar, Arnab Banerjee, Animesh Sahana, Ipsit Hauli, Subhra Kanti Mukhopadhyay, Damir A. Safin, Maria G. Babashkina, Michael Bolte, Yann Garcia and Debasis Das
Dalton Trans., 2013, DOI: 10.1039/C3DT50450F
Free to access until 13th May
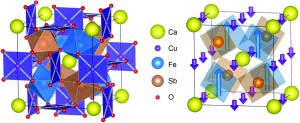
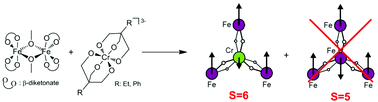
Frustration relieved ferrimagnetism in novel A- and B-site-ordered quadruple perovskite
Wei-tin Chen, Masaichiro Mizumaki, Takashi Saito and Yuichi Shimakawa
Dalton Trans., 2013, DOI: 10.1039/C3DT50489A
Free to access until 13th May
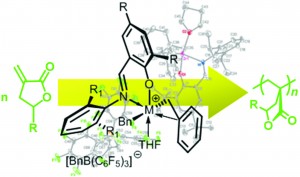
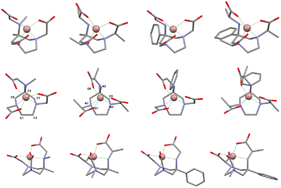
Chiral and achiral (imino)phenoxy-based cationic group 4 non-metallocene complexes as catalysts for polymerization of renewable α-methylene-γ-butyrolactones
Ravikumar R. Gowda and Eugene Y.-X. Chen
Dalton Trans., 2013, DOI: 10.1039/C3DT50430A
Free to access until 30th April