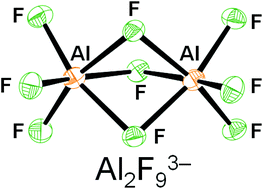
Fluorides of aluminium are used in the aluminium industry and also as catalysts for ozone friendly alternatives to chlorofluorocarbons (CFC’s). Previously, crystal structures of some fluoroaluminate anions have been solved by the introduction of either organic or metal cations. But until now, the crystal structure of a particular fluoroaluminate anion, the bioctahedral Al2F93- anion had not been reported.
The crystal structure of this anion has recently been solved by Rika Hagiwara and colleagues at Kyoto University. They first synthesized the compound, [C18MIm][AlF4] (1-methyl-3-octadecylimidazolium tetrafluoroaluminate) and discovered that slow evaporation of the chloroform solution gave transluscent crystals of [C18MIm]3[Al2F9](CH2Cl2)n. It is thought that this species was formed by the reaction below:
3[C18MIm][AlF4] + nCH2Cl2 → [C18MIm]3[Al2F9](CH2Cl2)n + AlF3
The Al2F93- anion has two octahedral AlF6 units which are face-sharing and has D3h symmetry.
To find out more, read the full Dalton Transactions article:
The first crystallographic example of a face-sharing fluoroaluminate anion Al2F93−
Fei Xu, Kazuhiko Matsumoto and Rika Hagiwara