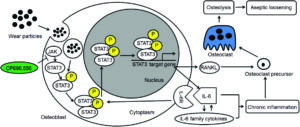
Total hip arthroplasty (THA) is required in orthopaedic surgery mostly for treating end stage joint disease. A good number of patients (9.1%) require revision surgeries due to aseptic loosening, which means osteolysis around the prostheses caused by the generation of wear particles. Now, there are two established mechanisms to understand wear induced osteolysis: wear particles produce inflammatory cytokines and activated osteoclasts; and wear particles disturb the differentiation, survival and function of osteoblasts and osteoclasts. However, the mechanism of interaction between osteoblasts and osteoclasts and the influence of wear particles is not clearly understood.
Researchers from China sought to analyze the role of interleukin-6 (IL-6) dependent inflammatory response in osteoblasts treated with TiAl6V4 nanoparticles (TiPs) and also the protective action of IL-6/STST3 (an important transcription factor) inhibition. TiPs obtained from the prosthesis of a patient with aseptic loosening were characterized for shape, size distribution, chemical composition, etc.
As measured by real time-PCR, the expression of IL-6, IL-11, LIF and OSM all increased when MC3T3-E1 cells were stimulated by TiPs in vitro as well as in the periosteum of mice. It was also found via western blotting that the protein levels of activated STAT3 were upregulated following treatment of cells with TiPs in a time and dose dependent fashion. This corroborated the immunofluorescence staining results both in vitro and in vivo, thereby confirming that TiPs activate STAT3 expression in osteoblasts. By using CP690,550 as an inhibitor of STAT3 activation, it was found that upregulation of IL-6- and IL-6-dependent inflammatory cytokine expression was reduced.
Next, using real-time PCR, it was shown that mRNA expression of RANKL, an indicator of osteoclastogenesis increased 13 fold in osteoblasts stimulated by TiPs. Using micro-CT with 3-dimensional reconstruction and quantitative analysis of bone parameters, it was confirmed that inhibition of the STAT3/ IL-6 pathway by inhibitor CP690,550 resulted in suppression of TiP induced osteolysis.
All these results taken together suggest that TiPs induced activation of STAT3 which led to osteolysis by promoting inflammation in osteoblasts and activating osteoclasts and that the inhibition of STAT3 activation by CP690,550 (tofacitinib) significantly reduced the activation of osteoclasts and protected against osteolysis via the STAT3/IL-6 signalling pathway. This suggests use of tofacitinib as a potential therapy for aseptic loosening.
To find out more please read:
Biomaterials Science, 2020, DOI: 10.1039/D0BM01256D
About the web writer:
 Saswat Choudhury is a graduate student at the Indian Institute of Science Bangalore pursuing research on biomaterials and tissue engineering. He studies bioabsorbable polymers, design and characterization for biomedical applications. Besides research, he is also interested in science communication. You can find him on Twitter @saswatchoudhur1
Saswat Choudhury is a graduate student at the Indian Institute of Science Bangalore pursuing research on biomaterials and tissue engineering. He studies bioabsorbable polymers, design and characterization for biomedical applications. Besides research, he is also interested in science communication. You can find him on Twitter @saswatchoudhur1