Alzheimer’s disease (AD) presently occupies the topmost position among the most commonly diagnosed neurodegenerative diseases worldwide with the number of affected people forecasted to reach 100 million by 2050. It is characterized by progressive memory loss, impairment of cognitive function, and inability to perform activities of daily life. The key to understanding AD lies in developing effective models which should ideally recapitulate all aspects of the disease. Furthermore, high inaccessibility to the human brain makes it desirable to study neuronal function and degeneration using appropriate in vivo or in vitro model systems of brain cultures. Increasing evidence indicates the superiority of three-dimensional (3D) in vitro cell culture platforms over conventional two-dimensional (2D) monolayer cultures in mimicking native in vivo microenvironments.
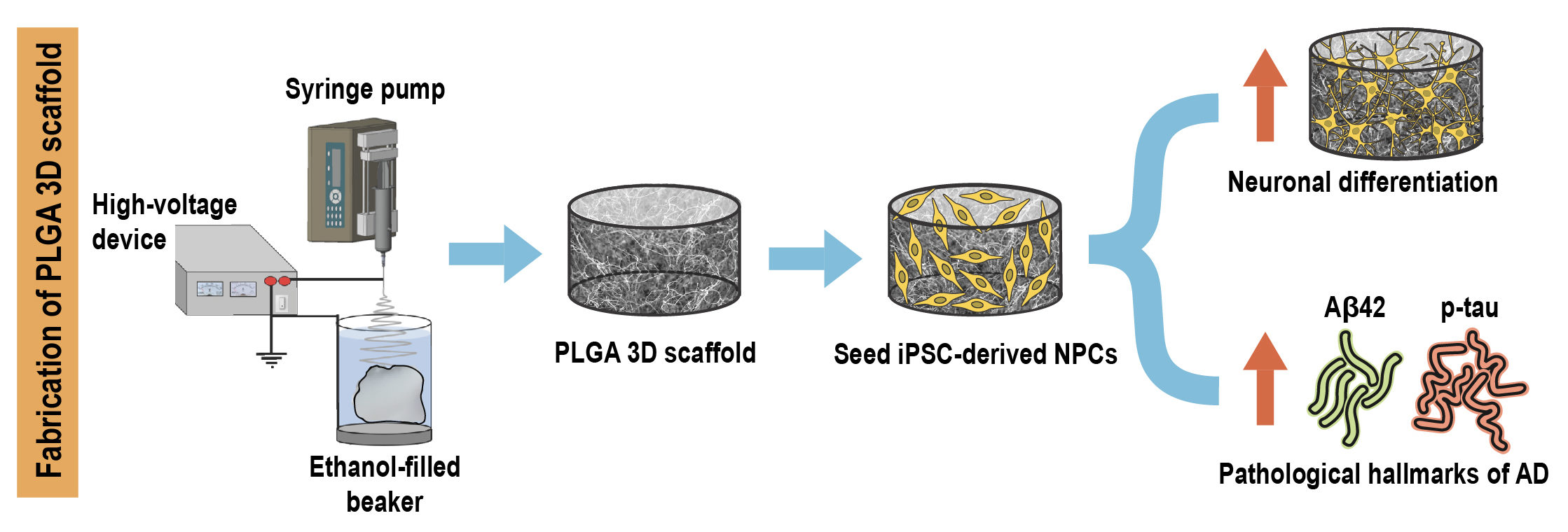
Researchers from Singapore have recently developed a novel 3D in vitro model of AD by encapsulating patient induced pluripotent stem cell (iPSC) derived neural progenitors in poly(lactic-co-glycolic acid) (PLGA) microtopographic scaffolds fabricated using wet electrospinning. They demonstrate that 3D culture robustly recapitulates and accelerates early-stage AD pathogenesis compared with Petri dish-based 2D monolayer controls.
First, they achieved deep cellular infiltration and uniform distribution inside the 3D microfibrous scaffold by optimizing various parameters such as fiber diameter, pore size, porosity and hydrophilicity. The stiffness of the microfiber scaffold was found to be comparable to the elasticity of native brain tissue, indicating its capability to promote realistic physiological responses.
Next, they compared key neural stem cell features including viability, proliferation and differentiation in 3D culture with 2D monolayer controls. The 3D microfibrous substrate reduced cell proliferation and significantly accelerated neuronal differentiation within just seven days of culture.
Finally, they demonstrated that 3D scaffold-based culture spontaneously enhanced pathogenic amyloid-beta 42 (Aβ42) and phospho-tau levels in differentiated neurons carrying familial AD (FAD) mutations compared with age-matched healthy controls. More importantly, recapitulation of both pathologies was more pronounced and consistent in 3D culture compared with the same cell lines in 2D monolayer culture conditions.
Taken together, the results indicate that the tunable scaffold-based 3D neuronal culture platform serves as a suitable in vitro model that robustly recapitulates and accelerates pathogenic characteristics of FAD-iPSC derived neurons. It can also be extended to model other complex neurodegenerative diseases and to evaluate prospective therapeutic candidates.
To find out more please read:
A microfiber scaffold-based 3D in vitro human neuronal culture model of Alzheimer’s disease
Vivek Damodar Ranjan, Lifeng Qiu, Jolene Wei-Ling Lee, Xuelong Chen, Se Eun Jang, Chou Chai, Kah-Leong Lim, Eng-King Tan, Yilei Zhang, Wei Min Huang and Li Zeng
Biomaterials Science, 2020, DOI: 10.1039/D0BM00833H