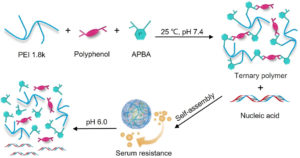
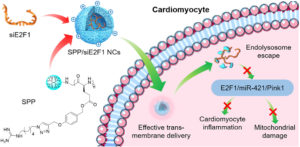
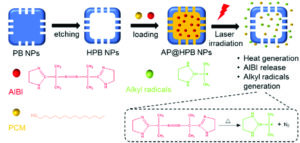
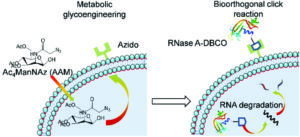
It is with great pleasure that we announce Li Tang (EPFL) as the recipient of the 2025 Biomaterials Science lectureship.
This award, established in 2014, honours an early-career researcher who has made significant contribution to the biomaterials field. The recipient is selected by the Biomaterials Science Editorial Board from a list of candidates nominated by the community.
Read Li’s Biomaterials Science publications for FREE until 31st August. These and articles from our previous lectureship winners can be found in our lectureship winners collection.
Read our interview with Li below:
How has your research evolved from your first article to this most recent article?
My research has evolved significantly over time. I began with a focus on material synthesis and nanotechnology during my bachelor and PhD studies, where I explored nanomaterial drug delivery systems aiming to improve cancer targeting and therapeutic efficiency. During my postdoc training, I got fascinated by the field of immunoengineering and developed the immune cell therapy with nanoparticles “backpacks”. Upon establishing my independent laboratory at EPFL, my lab’s work has concentrated on cancer immunology and immunotherapy, integrating my previous experience to develop innovative mechanical and metabolic engineering strategies that enhance immune responses against tumors.
What excites you most about your area of research and what has been the most exciting moment of your career so far?
What excites me most about my research area is the potential to make new discoveries that can directly impact patient outcomes. In cancer immunotherapy field, every breakthrough brings us closer to more effective treatments. The possibility of translating scientific findings into real-world therapies that can improve or even save lives is incredibly motivating.
As for the most exciting moment in my career so far, it was when our team observed a strong therapeutic response in cancer patients treated with the immunotherapy we invented. Seeing the direct impact of our work validated our approach and reinforced my passion for this field.
In your opinion, what are the most important questions to be asked/answered in your field of research?
One of the most critical questions in my field is how we can effectively translate biomaterials research into real clinical impact. While we’ve made significant advances in designing sophisticated materials, the key challenge lies in ensuring they are not only effective and safe in experimental models but also practical and scalable for clinical use. I believe bridging the gap between bench and bedside—making sure our innovations reach patients and improve outcomes—is what will define the future of the field.
How do you feel about Biomaterials Science as a place to publish research on this topic?
Biomaterials Science is an excellent journal for publishing research in this area. It has a strong reputation for featuring high-quality, interdisciplinary work that bridges materials science, biology, and medicine. The journal’s focus on translational potential makes it especially suitable for studies aiming to make a clinical impact, such as those involving biomaterials for drug delivery or cancer immunotherapy. Publishing in Biomaterials Science ensures visibility within the biomedical communities, and it encourages critical dialogue across related fields.
Which of your Biomaterials Science publications are you most proud of and why?
In this article, we reported a novel nanoparticle “backpack” strategy to enhance adoptive T cell therapy for cancer. Plus, I was invited to contribute this article as an “Emerging Investigator” in this issue.
In which upcoming conferences or events (online or in person) may our readers meet you?
Biomaterials and Tissue Engineering Conference GRC 2025
34th Annual Conference of the European Society for Biomaterials 2025
Cellular and Humoral Immunotherapy in Autoimmunity (CHIA 2025)
17th Annual Protein & Antibody Engineering Summit (PEGS 2025)
Can you share one piece of career-related advice or wisdom with early career scientists?
My advice would be to focus your efforts on high-impact research—work that either addresses fundamental scientific questions or has the potential for real societal benefit. Focusing on advancing core knowledge or solving pressing real-world problems, will not only make your research more meaningful but also help build a fulfilling and lasting career.
How do you spend your spare time?
In my spare time, I enjoy staying active through jogging, hiking, and skiing. I also value spending quality time with my family—whether it’s exploring nature together or simply relaxing at home.
We would like to thank everybody who nominated a candidate for the 2025 Biomaterials Science Lectureship. The Editorial Board had a very difficult task in choosing a winner from the many excellent and worthy candidates.
Please join us in congratulating Li on winning this award!














 Hang Ping received his PhD degree in materials processing engineering (2017) from the Wuhan University of Technology. He is currently a professor in Wuhan University of Technology. His research interests mainly focus on bioprocessing-inspired synthesis and fabrication. He has published more than 50 peer-reviewed papers as coauthors, authorized 6 invention patents in China, and hosted 3 projects from national natural science foundation of China.Hang received an award sponsored by Materials Horizons for his talk entitled ‘Bioprocessing-inspired intrafibrillar collagen mineralization with megapascal contractile prestresses’
Hang Ping received his PhD degree in materials processing engineering (2017) from the Wuhan University of Technology. He is currently a professor in Wuhan University of Technology. His research interests mainly focus on bioprocessing-inspired synthesis and fabrication. He has published more than 50 peer-reviewed papers as coauthors, authorized 6 invention patents in China, and hosted 3 projects from national natural science foundation of China.Hang received an award sponsored by Materials Horizons for his talk entitled ‘Bioprocessing-inspired intrafibrillar collagen mineralization with megapascal contractile prestresses’ Jan Grzelak was born in 1993 in Warsaw, Poland. He obtained his bachelor´s (B.Sc.) and master´s degrees (M.Sc.) in Nanostructure Engineering from University of Warsaw. In 2017, he was granted a “la Caixa” INPhINIT Fellowship to pursue a PhD degree in Materials Science at Institut de Ciència de Materials de Barcelona (ICMAB-CSIC), under the supervision of Prof. Anna Roig Serra and Dr. Martí Gich Garcia, in the Nanoparticles and Nanocomposites Group. In October 2021, he obtained his PhD with Cum Laude qualification for his doctoral thesis entitled “Magnetic and fluorescent mesoporous silica nanorods towards liver theranostic uses”. Later, he pursued an additional master’s degree in Biomedicine at the University of Barcelona. In January 2023, he started working as a postdoctoral researcher in the ERC project “Bio-inspired AntiMicrobial Bone Bioceramics: Deciphering contact-based biocidal mechanisms” (BAMBBI) in the research line of Biomaterials for Bone Regeneration led by Prof. Maria Pau Ginebra at the Polytechnic University of Catalonia (UPC), where he works on the development of calcium phosphate surfaces with tailored nanotopographies.
Jan Grzelak was born in 1993 in Warsaw, Poland. He obtained his bachelor´s (B.Sc.) and master´s degrees (M.Sc.) in Nanostructure Engineering from University of Warsaw. In 2017, he was granted a “la Caixa” INPhINIT Fellowship to pursue a PhD degree in Materials Science at Institut de Ciència de Materials de Barcelona (ICMAB-CSIC), under the supervision of Prof. Anna Roig Serra and Dr. Martí Gich Garcia, in the Nanoparticles and Nanocomposites Group. In October 2021, he obtained his PhD with Cum Laude qualification for his doctoral thesis entitled “Magnetic and fluorescent mesoporous silica nanorods towards liver theranostic uses”. Later, he pursued an additional master’s degree in Biomedicine at the University of Barcelona. In January 2023, he started working as a postdoctoral researcher in the ERC project “Bio-inspired AntiMicrobial Bone Bioceramics: Deciphering contact-based biocidal mechanisms” (BAMBBI) in the research line of Biomaterials for Bone Regeneration led by Prof. Maria Pau Ginebra at the Polytechnic University of Catalonia (UPC), where he works on the development of calcium phosphate surfaces with tailored nanotopographies.