According to the World Health Organization, cardiovascular disease causes 17.3 million deaths worldwide, with projections reaching 23.3 million deaths by the year 2030. Unfortunately, heart attack patients still have limited therapeutic options, commonly relying on left ventricular assist devices (LVADs) and heart transplantation. To provide more modern therapies, physicians have turned to tissue engineers to develop biomaterials that enable local regeneration of the heart to restore function and ideally improve the quality of life for the patient.
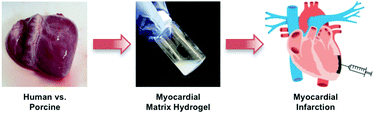
Professor Karen Christman’s lab at the University of California – San Diego (UCSD) is exploring methods to fabricate injectable hydrogels for cardiac repair. In the present study, the lab has developed human myocardial matrix (HMM), and compared the material properties of HMM to previously fabricated porcine myocardial matrix (PMM). The materials are made directly from the decellularized extracellular matrix (ECM) from human and porcine hearts. The decellularized matrices are composed of the natural structural proteins found in the heart, which make the ideal environment needed to promote cardiac cell growth and maturation.
To produce HMM, seven human hearts with a patient age range of 41-69 years were decellularized using sodium dodecyl sulfate (SDS) and a series of lyophilization, milling, and digestion steps. Due to the dramatic patient-to-patient variability between hearts, over 50% of the HMM solutions were not able to self-assemble into hydrogels at physiological conditions, unlike self-assembling PMM hydrogels. The irreproducibility of HMM hydrogel fabrication is likely due to differences in protein composition between HMM and PMM. Using mass spectrometry to identify the proteins present in the decellularized matrices, the authors showed that porcine and human hearts have inherent differences in their matrix composition.
Although HMM did not produce hydrogels reproducibly, the matrix was still useful for in vitro cell culture protocols. Using PMM and HMM as coatings for cell culture plates, increased proliferation of rat aortic smooth muscle cells (RASMCs) and human coronary artery endothelial cells (HCAECs) was observed on HMM coated plates compared to PMM coated plates. Additionally, cell cultured on both HMM and PMM matrices showed increased expression of early cardiac transcription factor markers in human fetal cardiomyocyte progenitor cells (hCMPCs). This result indicates that biochemical cues from the HMM and PMM proteins may enhance early stages of cardiomyocyte differentiation.
Even though HMM was shown to not be a likely candidate for clinical translation due to large variability between samples, PMM injectable hydrogels are still a promising alternative for improving cardiac repair in vivo. Additionally, HMM materials may be used for future in vitro cell culture and cardiomyocyte differentiation protocols.
Human versus porcine tissue sourcing for an injectable myocardial matrix hydrogel
Todd D. Johnson, Jessica A. DeQuach, Roberto Gaetani, Jessica Ungerleider, Dean Elhag, Vishal Nigam, Atta Behfar, and Karen L. Christman
Biomater. Sci., 2014, Advance Article, DOI: 10.1039/C3BM60283D
Brian Aguado is currently a Ph.D. Candidate and NSF Fellow in the Biomedical Engineering department at Northwestern University. He holds a B.S. degree in Biomechanical Engineering from Stanford University and a M.S. degree in Biomedical Engineering from Northwestern University. Read more about Brian’s research publications here.
To keep up-to-date with all the latest research, sign-up to our RSS feed or Table of contents