Protein-based biologics are gaining tremendous interests in the biomedical field after several successful translations into commercialized products. To this end, key hurdles in the development of protein-based therapeutics need to be properly addressed first. One of the most prominent issues is the instability of protein therapeutics in vivo, which requires higher and more frequent doses. As a result, protein-based therapy can become prohibitive in cost and may pose potential safety risks. Engineering protein therapeutics using biomaterials offer unique strategies to solve this problem by prolonging the half-life and enhancing the efficacy of these. In this blog, we feature three articles published in Biomaterials Science recently on the topic of Engineering Advanced Biologics as therapeutic agents.
1. Multivalent conjugates of basic fibroblast growth factor enhance in vitro proliferation and migration of endothelial cells
Aline Zbinden, Shane Browne, Eda I. Altiok, Felicia L. Svedlund, Wesley M. Jackson and Kevin E. Healy
Biomater. Sci., 2018, Advance Article, DOI: 10.1039/C7BM01052D
The authors reported the chemical conjugation of basic fibroblast growth factor (bFGF) onto hyaluronic acid (HA). Such multivalent conjugates were envisioned to increase residence time in vivo, protect bFGF from enzymatic degradation, and enhance protein bioactivity. Using a small molecule linker (N-(ε-Maleimidocaproic acid) hydrazide), bFGF was successfully conjugated at two different protein-to-polymer ratios and their structures were confirmed by extensive physico-chemical characterization. Compared to bFGF alone, HA conjugated bFGF displayed enhanced activity to promote the proliferation and more interestingly, the scratch closure of human umbilical cord vein endothelial cells. It’s noteworthy that the protein-to-polymer ratio and the conjugate size are two key parameters that could be easily tuned to modulate the bioactivity of the conjugate.
2. Synergistic effects of hyaluronate – epidermal growth factor conjugate patch on chronic wound healing
Yun Seop Kim, Dong Kyung Sung, Won Ho Kong, Hyemin Kim and Sei Kwang Hahn
Biomater. Sci., 2018, Advance Article, DOI: 10.1039/C8BM00079D
Similar to the strategy discussed above, the authors also utilized the biocompatible HA as carrier to conjugate epidermal growth factor (EGF) which is otherwise labile in vivo. The enzymatic degradation and stability studies revealed the superior stability of EGF when conjugated with HA. In vitro, HA–EGF conjugate increased keratinocyte proliferation, VEGF secretion, and migration towards scratch closure when compared to EGF alone. In vivo, HA–EGF conjugates loaded HA patch closed the wound in a rat model by enhancing de novo ECM secretion and mitigating inflammatory response. Overall, those comprehensive studies demonstrated the significance of protein-HA conjugates in improving the therapeutic effects of protein biologics.
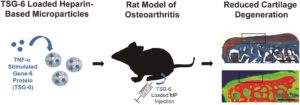
3. Intra-articular TSG-6 delivery from heparin-based microparticles reduces cartilage damage in a rat model of osteoarthritis
Liane E. Tellier, Elda A. Treviño, Alexandra L. Brimeyer, David S. Reece, Nick J. Willett, Robert E. Guldbergde and Johnna S. Temenoff
Biomater. Sci., 2018, Advance Article, DOI: 10.1039/C8BM00010G
Here, the authors aimed to improve the delivery of TNF-α-stimulated gene-6 (TSG-6) for treating osteoarthritis. Specifically, they focused on heparin, another glycosaminoglycan, as a carrier biomaterial for TSG-6 delivery. It was first discovered that N-desulfated heparin maintained the beneficial role in promoting the bioactivity of TSG-6. Microparticles were thus made from N-desulfated heparin followed by TSG-6 encapsulation. TSG-6 was able to be released and demonstrated significantly higher anti-plasmin activity compared to soluble TSG-6. In a rat medial meniscal transection model, treatment of TSG-6 delivered by N-desulfated heparin but not in soluble form resulted in similar cartilage thickness, volume and GAG level compared to uninjured cartilage. These results showcased the advantages of heparin based microparticle in preserving and enhancing the bioactivity of therapeutic TSG-6 protein.
All articles free to read until 24 May
About the webwriter
 Yingfei Xue is a web writer for Biomaterials Science. Currently, he is a PhD candidate and graduate student researcher in Dr. Shilpa Sant lab at the University of Pittsburgh, USA. His research focus on nano-/micro-technology in novel heart valve therapy. Find him on Twitter: @Phil_Xue or connect him on ResearchGate
Yingfei Xue is a web writer for Biomaterials Science. Currently, he is a PhD candidate and graduate student researcher in Dr. Shilpa Sant lab at the University of Pittsburgh, USA. His research focus on nano-/micro-technology in novel heart valve therapy. Find him on Twitter: @Phil_Xue or connect him on ResearchGate