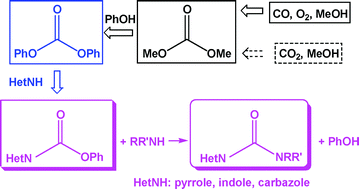
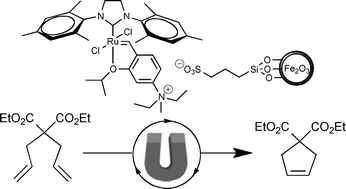
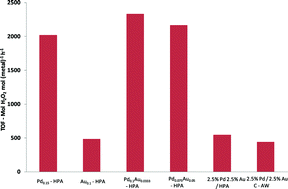
This month sees the following articles in Green Chemistry that are in the top ten most accessed:-
Industrial biotechnology-the future of green chemistry?
Stefanie Wenda, Sabine Illner, Annett Mell and Udo Kragl
Green Chem., 2011, 13, 3007-3047, DOI: 10.1039/C1GC15579B
Recent advances in ionic liquid catalysis
Qinghua Zhang, Shiguo Zhang and Youquan Deng
Green Chem., 2011, Advance Article, DOI: 10.1039/C1GC15334J
Extracting wood lignin without dissolving or degrading cellulose: investigations on the use of food additive-derived ionic liquids
André Pinkert, Dagmar F. Goeke, Kenneth N. Marsh and Shusheng Pang
Green Chem., 2011, 13, 3124-3136, DOI: 10.1039/C1GC15671C
Green synthesis of metal nanoparticles using plants
Siavash Iravani
Green Chem., 2011, Advance Article, DOI: 10.1039/C1GC15386B
A facile method for the recovery of ionic liquid and lignin from biomass pretreatment
Dean C. Dibble, Chenlin Li, Lan Sun, Anthe George, Aurelia Cheng, Özgül Persil Çetinkol, Peter Benke, Bradley M. Holmes, Seema Singh and Blake A. Simmons
Green Chem., 2011, 13, 3255-3264, DOI: 10.1039/C1GC15111H
Efficient catalytic conversion of fructose into hydroxymethylfurfural by a novel carbon-based solid acid
Jianjian Wang, Wenjie Xu, Jiawen Ren, Xiaohui Liu, Guanzhong Lu and Yanqin Wang
Green Chem., 2011, 13, 2678-2681, DOI: 10.1039/C1GC15306D
Ethyl lactate as a solvent: Properties, applications and production processes – a review
Carla S. M. Pereira, Viviana M. T. M. Silva and Alírio E. Rodrigues
Green Chem., 2011, 13, 2658-2671, DOI: 10.1039/C1GC15523G
Stabilization of Cu(0)-nanoparticles into the nanopores of modified montmorillonite: An implication on the catalytic approach for ‘Click’ reaction between azides and terminal alkynes
Bibek Jyoti Borah, Dipanka Dutta, Partha Pratim Saikia, Nabin Chandra Barua and Dipak Kumar Dutta
Green Chem., 2011, Advance Article, DOI: 10.1039/C1GC16021D
An active and stable CaO-CeO2 catalyst for transesterification of oil to biodiesel
W. Thitsartarn and S. Kawi
Green Chem., 2011, Advance Article, DOI: 10.1039/C1GC15596B
Reusable ammonium salt-tagged NHC-Cu(i) complexes: preparation and catalytic application in the three component click reaction
Wenlong Wang, Junliang Wu, Chungu Xia and Fuwei Li
Green Chem., 2011, Advance Article, DOI: 10.1039/C1GC15871F
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to Green Chemistry? Then why not submit to us today or alternatively email us your suggestions.