This month sees the following articles in Green Chemistry that are in the top ten most accessed:-
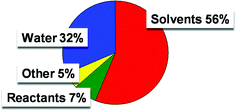
Searching for green solvents
Philip G. Jessop
Green Chem., 2011, Advance Article, DOI: 10.1039/C0GC00797H, Perspective
Enzyme-mediated oxidations for the chemist
Frank Hollmann, Isabel W. C. E. Arends, Katja Buehler, Anett Schallmey and Bruno Bühler
Green Chem., 2011, 13, 226-265, DOI: 10.1039/C0GC00595A, Critical Review
Greener solvents for ruthenium and palladium-catalysed aromatic C-H bond functionalisation
Cedric Fischmeister and Henri Doucet
Green Chem., 2011, Advance Article, DOI: 10.1039/C0GC00885K, Critical Review
Synthesis of highly stable dispersions of nanosized copper particles using l-ascorbic acid
Jing Xiong, Ye Wang, Qunji Xue and Xuedong Wu
Green Chem., 2011, Advance Article, DOI: 10.1039/C0GC00772B, Paper
Tertiary amine solvents having switchable hydrophilicity
Philip G. Jessop, Lisa Kozycz, Zahra Ghoshouni Rahami, Dylan Schoenmakers, Alaina R. Boyd, Dominik Wechsler and Amy M. Holland
Green Chem., 2011, 13, 619-623, DOI: 10.1039/C0GC00806K, Paper
Supported ionic liquid silica nanoparticles (SILnPs) as an efficient and recyclable heterogeneous catalyst for the dehydration of fructose to 5-hydroxymethylfurfural
Kalpesh B. Sidhpuria, Ana L. Daniel-da-Silva, Tito Trindade and João A. P. Coutinho
Green Chem., 2011, 13, 340-349, DOI: 10.1039/C0GC00690D, Paper
Synthesis of sugar alcohols by hydrolytic hydrogenation of cellulose over supported metal catalysts
Hirokazu Kobayashi, Yukiko Ito, Tasuku Komanoya, Yuto Hosaka, Paresh L. Dhepe, Koji Kasai, Kenji Hara and Atsushi Fukuoka
Green Chem., 2011, 13, 326-333, DOI: 10.1039/C0GC00666A, Paper
CuCl-catalyzed green oxidative alkyne homocoupling without palladium, ligands and bases
Kun Yin, Chunju Li, Jian Li and Xueshun Jia
Green Chem., 2011, 13, 591-593, DOI: 10.1039/C0GC00413H, Communication
An efficient activity ionic liquid-enzyme system for biodiesel production
Teresa De Diego, Arturo Manjón, Pedro Lozano, Michel Vaultier and José L. Iborra
Green Chem., 2011, 13, 444-451, DOI: 10.1039/C0GC00230E, Paper
Converting carbohydrates to bulk chemicals and fine chemicals over heterogeneous catalysts
Maria J. Climent, Avelino Corma and Sara Iborra
Green Chem., 2011, 13, 520-540, DOI: 10.1039/C0GC00639D, Critical Review
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to Green Chemistry? Then why not submit to us today or alternatively email us your suggestions.