Thermoelectric materials enable the direct conversion of thermal to electric energy, and as such, have received considerable attention as a source of sustainable clean energy. The performance of a thermoelectric material is characterized by the dimensionless figure of merit, zT = S2σT/κ, where S is the Seebeck coefficient, σ is the electrical conductivity, κ is the thermal conductivity, and T is the absolute temperature. Achieving high zT requires careful design of low thermal conductivity and high power factor (PF = S2σ). Typically, thermoelectric materials with a high zT are heavily doped semiconductors, which have been extensively studied at medium and high temperatures, but less so at near room temperature. Recently, orthorhombic Ag2Se has attracted much interest for near-room-temperature thermoelectric applications as they are anticipated to catalyze tremendous growth in energy harvesting for advancing internet of things appliances, self-powered wearable medical systems, and self-powered wearable intelligent devices. In order to optimize the thermoelectric performance of orthorhombic Ag2Se, it is vital to understand the correlation between composition, structure, and transport properties. A variety of methods have been successfully developed for the preparation of Ag2Se thermoelectric materials, including high-temperature solid-state reactions, room-temperature grinding, high‐energy mechanical milling, and pulsed hybrid reactive magnetron sputtering techniques. In comparison, solution‐based approaches are relatively less investigated for the synthesis of Ag2Se, though widely used for generating CdSe, ZnSe and Cu2-xSe compounds, as these methods offer the unique advantage of excellent control over material stoichiometry with high production throughputs at ambient conditions.
Recently, researchers at the Institute of Materials Research and Engineering (IMRE), Agency for Science, Technology and Research (A*STAR), and Tianjin University, demonstrated a high ZT value of near unity at near-room-temperature through compositionally tuned hybridization of n-type Ag0:Ag2Se (Fig. 1). A series of n-type Ag0:Ag2Se materials has been systematically prepared through a surfactant-free, aqueous solution-based approach under ambient conditions. This strategy enables fine control over phases and compositions through nanoscale hybridization, yet remains applicable to large-scale production methods. By prolonging reaction times, the synthetic process is carefully developed/optimized to adjust the stoichiometry of Ag and Se by modulating the oxidation states of Ag and Se in the reaction medium, producing a series of Ag0:Ag2Se (Ag0 excess at 50.86%, 45.80%, 15.97%, 6.10%, 4.31% and 1.96%) with enhanced thermoelectric properties.
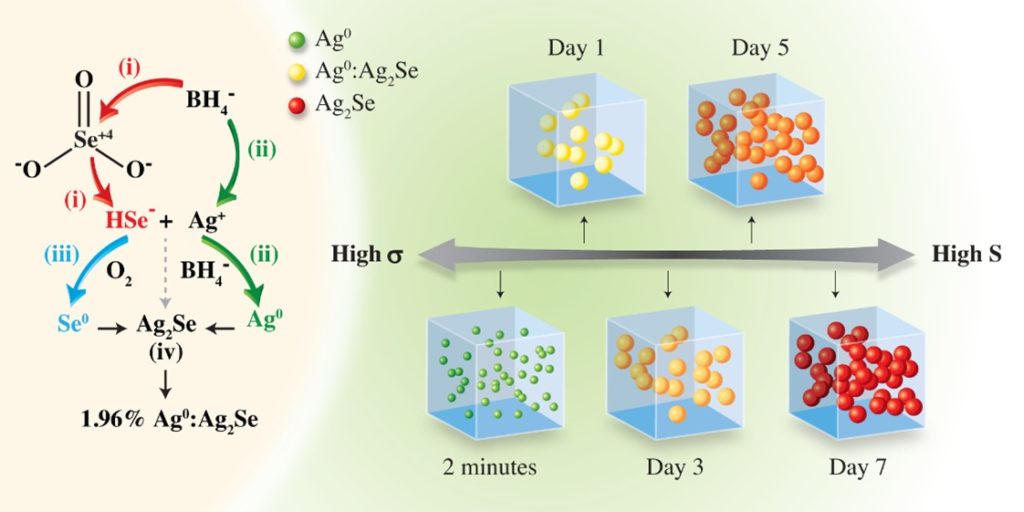
Fig. 1 Schematic synthesis of Ag0:Ag2Se hybrids for a period of 7 days at room temperature under aqueous condition, with different molar ratios of Ag:Se.
After hot-processing the powder by spark plasma sintering, the temperature-dependent electrical conductivity of 45.80% Ag0:Ag2Se prepared by reaction for 1 day was significantly higher than the rest of the Ag0:Ag2Se samples, which relates to its augmented carrier concentration due to hybridization with more Ag0. When the reaction time was prolonged, more Ag2Se was converted from Ag0, resulting in a drastic decrease in electrical conductivity for all the Ag0:Ag2Se samples including 6.10%, 4.31%, and 1.96% Ag0 from 3, 5 and 7 days of reaction, respectively. The temperature-dependent trends in Seebeck coefficient of Ag0:Ag2Se are opposite to those of electrical conductivity. These attributes lead to the lowest power factor for 45.80% Ag0:Ag2Se, in comparison to the rest of the Ag0:Ag2Se samples (Fig. 2a). In conjunction with fine-grained structure, which effectively scattered phonons at grain boundaries, the optimal excessive Ag0 of 1.96% after 7 days of reaction exhibited a high ZT value of close to unity (Fig. 2b).
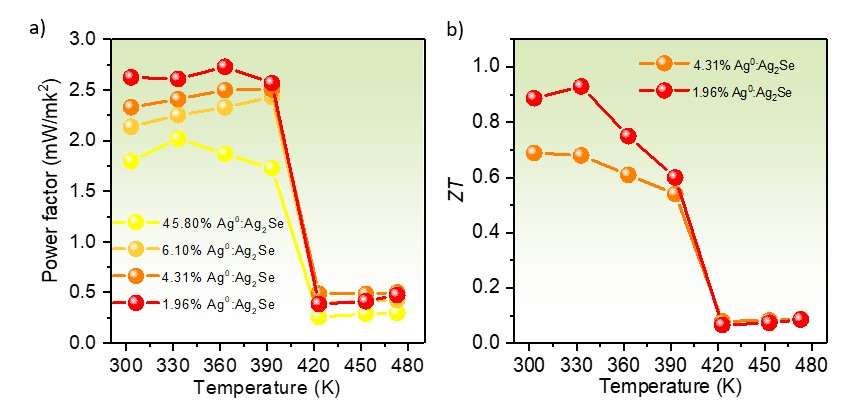
Fig. 2 (a) Temperature dependence of power factors of the hot- pressed pellets of 45.80%, 6.10%, 4.31%, and 1.96% Ag0:Ag2Se, synthesized after reactions for 1, 3, 5, and 7 days. (b) Temperature dependence of ZT value of the 1.96% Ag0:Ag2Se pellet in comparison with the 4.31% Ag0:Ag2Se pellet.
Instead of doping or alloying, our work presents an effective way to organize different nanoscale building blocks by precise hybridization at nanoscale, preserving the intrinsic properties of Ag2Se without incorporating different elements. On this basis, it would be of great interest in extending this solution strategy to the synthesis of hybridized multinary silver-based chalcogenides for further enhancing thermoelectric properties. Additionally, this solution approach could also find uses in the general synthesis of other metal chalcogenides, particularly useful for large-scale production.
Corresponding authors:
Tee Si Yin obtained her PhD in Biomedical Engineering from National University of Singapore. Currently, she is working as a research scientist at the Institute of Materials Research and Engineering, A*STAR. Her research focuses on the development of functional metal and semiconductor nanostructures for biomedical, environmental and energy applications.
Han Ming-Yong worked with IBM and Indiana University, followed by time spent at the National University of Singapore as a faculty member, before his current appointments with the Institute of Materials Research and Engineering and Tianjin University. His research addresses problems at the interfaces of nanoscience, nanotechnology, biotechnology and energy/biomedical applications. He has published >220 papers and filed >100 patents including national entries, with ~28,000 citations and >300 research highlights. He is a Fellow of the Royal Society of Chemistry (FRSC) and a Web of Science / Scopus highly cited researcher.