Controllable defects and interface engineering are perceived as promising routes to develop efficient noble-metal-free electrocatalysts for oxygen evolution reaction (OER), the bottleneck of overall water splitting. Recently Metal-organic frameworks (MOFs) have received particular attention for their remarkable OER performance because of their vast surface area, ability to tune porosity, and functionalization with mixed metals/ligands. Among various MOFs, Prussian blue analogs (PBAs) have been extensively studied for their potential in catalyzing the OER process. However, pristine PBA cubes suffer from low conductivity and exhibit insufficient OER activity, resulting in high overpotential and limiting their OER electrocatalytic applicability.
PBAs’ components, topologies, and surface engineering can be modified to improve their catalytic properties to address these limitations. One common structural alteration is the introduction of vacancies (defects) within the PBA cubes to facilitate ion diffusion, often accomplished through chemical etching that can change the local electronic configuration to boost the OER kinetics. There are only a few works on the selective etching of PBAs to enhance their performance; therefore, modification of the PBAs by the chemical etching process is a trending topic in OER electrocatalyst designing.
Recently, the Zidki’s research group at Ariel University highlighted the significance of combining the PBA etching effect with the decoration of molybdenum disulfide (MoS2) on the edges and surfaces of Co-Fe PBA for catalyzing the OER kinetics. They proposed using an ethanol-water mixture as a mild etchant, eliminating the need for a capping or stabilizing agent.
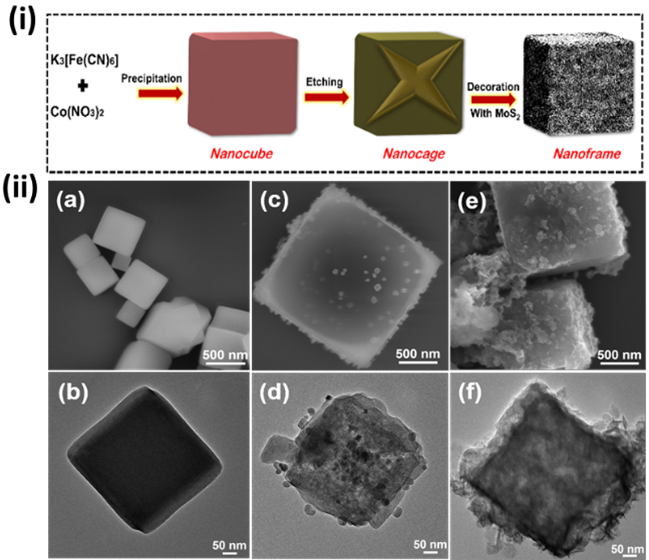
Figure 1. (i) Synthetic scheme of Etched-PBA-MoS2 nanoframes. (ii) HR-SEM images of PBA nanocubes (a), Etched-PBA nanocages (c), and Etched-PBA-MoS2 nanoframes (e), and their corresponding TEM images (b,d, and f).
The Etched-PBA-MoS2 nanoframes presented superior OER performance, requiring an overpotential as low as 260 mV on carbon cloth substrate to obtain the current density of 10 mA cm−2 with a corresponding Tafel slope of 55 mV dec-1 (Fig. 2a and b). The Etched-PBA-MoS2 nanoframes also outperform with a lower charge transfer resistance (Rct) value (smallest semicircle), indicating its faster charge-transfer kinetics (Fig. 2c). The catalyst retains its catalytic activity for a long-term stability test, proving its OER in a real-time application (Fig. 2d).

Figure 2. (a) LSV curves; (b) Tafel plots; (c) EIS-Nyquist plots measured at 1.5 V vs. RHE in 1.0 M KOH; (d) OER stability test of Etched-PBA-MoS2/CC.
This work demonstrates that the excellent electrocatalytic activity arises from two primary factors: (1) hollowing PBA nanocubes by the etching process increases the density of active sites to promote mass transport; (2) binding MoS2 on the surface of PBA nanocages induces a synergistic effect – the electronic interactions among the active components tune the electronic structures of Co, Fe, and Mo sites. Their work renders a feasible pathway to optimize the etching effect and fasten different metal sulfide heterostructures on PBAs to achieve an excellent OER performance.
Corresponding author:
Dr. Tomer Zidki received his Ph.D. degree in 2010 from the Chemistry Department, Ben-Gurion University, Beer Sheva, Israel, in the field of radical reactions with nanoparticle catalysts. He pursued postdoctoral research at the Brookhaven National Laboratory, NY, USA, where he gained experience in redox catalytic processes. Dr. Zidki is an Assistant Professor in the Chemical Sciences Department, Ariel University, Israel, where he leads the Nanoparticle Catalysts group. He is also the Head of the Linear Electron Accelerator Facility for fast chemical reactions. Dr. Zidki has guided ten Ph.D. and three M.Sc. students. His research focuses on redox catalyzed reactions by nanoparticles and photo- & electrocatalytic water splitting reactions using non-precious catalysts. In addition, Dr. Zidki’s group studies the kinetic mechanisms of redox reactions and radical reactions using the electron accelerator. Another field of interest of Dr. Zidki is Environmental Chemistry, in which he wrote two patents on nitrogen and sulfur oxides removal from flue gases.