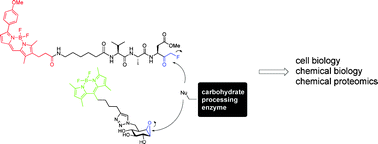
Work from Srinivas Hotha at the Indian Institute of Science Education & Research, and colleagues at the National Chemical Laboratory (Pune, India) features on the front cover of Issue 17. Their paper describes the efficient preparation of glycosylated amino acids using propargyl orthoesters as glycosyl donors, via gold-catalysis.
Are your interests in carbohydrate chemistry, or peptide and glycopeptide chemistry? Then this article is for you!
Facile synthesis of unusual glycosyl carbamates and amino acid glycosides from propargyl 1,2-orthoesters as glycosyl donors
Ashif Y. Shaikh, Gopalsamy Sureshkumar, Debasish Pati, Sayam Sen Gupta and Srinivas Hotha
Org. Biomol. Chem., 2011, 9, 5951-5959
DOI: 10.1039/C1OB05056G
As with all our cover articles, this paper is free to access for 6 weeks – why not download it today.












![GA[1]](https://blogs.rsc.org/ob/files/2011/08/GA12.gif)