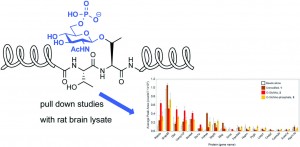
This month sees the following articles in Organic & Biomolecular Chemistry that are in the top ten most accessed:
Asymmetric organocatalytic formation of protected and unprotected tetroses under potentially prebiotic conditions
Laurence Burroughs, Paul A. Clarke, Henrietta Forintos, James A. R. Gilks, Christopher J. Hayes, Matthew E. Vale, William Wade and Myriam Zbytniewski
Org. Biomol. Chem., 2012, 10, 1565-1570
DOI: 10.1039/C1OB06798B
Total synthesis of (+)-anamarine
Krishnammagari Suresh Kumar and Cirandur Suresh Reddy
Org. Biomol. Chem., 2012, 10, 2647-2655
DOI: 10.1039/C2OB06940G
Olefin cross-metathesis for the synthesis of heteroaromatic compounds
Timothy J. Donohoe, John F. Bower and Louis K. M. Chan
Org. Biomol. Chem., 2012, 10, 1322-1328
DOI: 10.1039/C2OB06659A
Highly selective, naked-eye and fluorescent “off-on” probe for detection of histidine/histidine-rich proteins and its application in living cell imaging
Shenyi Zhang, Chunmei Yang, Weiping Zhu, Bubing Zeng, Youjun Yang, Yufang Xu and Xuhong Qian
Org. Biomol. Chem., 2012, 10, 1653-1658
DOI: 10.1039/C2OB06520G
Direct C–H cross-coupling approach to heteroaryl coumarins
Minsik Min, Bomi Kim and Sungwoo Hong
Org. Biomol. Chem., 2012, Advance Article
DOI: 10.1039/C2OB07137A
Bioinspired organocatalytic asymmetric reactions
Luca Bernardi, Mariafrancesca Fochi, Mauro Comes Franchini and Alfredo Ricci
Org. Biomol. Chem., 2012, Advance Article
DOI: 10.1039/C2OB07037E
Imidazole derivatives: A comprehensive survey of their recognition properties
Pedro Molina, Alberto Tárraga and Francisco Otón
Org. Biomol. Chem., 2012, 10, 1711-1724
DOI: 10.1039/C2OB06808G
An iterative Shimizu non-aldol approach for the stereoselective synthesis of C13-C22 fragment of callystatin A
Sandip A. Pujari and Krishna P. Kaliappan
Org. Biomol. Chem., 2012, 10, 1750-1753
DOI: 10.1039/C2OB06838A
Organocatalytic stereoselective synthesis of passifloricin A
Pradeep Kumar, Menaka Pandey, Priti Gupta and Dilip D. Dhavale
Org. Biomol. Chem., 2012, 10, 1820-1825
DOI: 10.1039/C2OB06711K
Direct preparation of thiazoles, imidazoles, imidazopyridines and thiazolidines from alkenes
Timothy J. Donohoe, Mikhail A Kabeshov, Akshat H. Rathi and Ian E. D. Smith
Org. Biomol. Chem., 2012, 10, 1093-1101
DOI: 10.1039/C1OB06587D
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to Organic & Biomolecular Chemistry? Then why not submit to us today or alternatively email us your suggestions.
Comments Off on Top ten most accessed articles in January
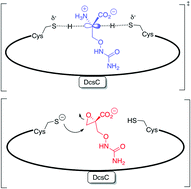
![GA[11]](https://blogs.rsc.org/ob/files/2012/03/GA111.gif) A sensing system to detect cysteine (Cys), an essential amino acid, has been developed by scientists in the US and China. Elevated levels of Cys have been associated with neurotoxicity, while Cys deficiency is involved in a number of other disorders.
A sensing system to detect cysteine (Cys), an essential amino acid, has been developed by scientists in the US and China. Elevated levels of Cys have been associated with neurotoxicity, while Cys deficiency is involved in a number of other disorders.