In this OBC ‘Hot paper’ John C. Vederas and coworkers describes studies on a key enzyme (DscC) in the biosynthesis of the important antibiotic D-cycloserine – commonly used against Mycobacterium tuberculosis when resistance to other antibiotics is observed.
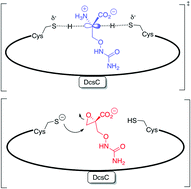 The enzyme is proposed to be an epimerase based on sequence similarity to a known enzyme DapF (diaminopimelate epimerase), and the authors show that DscC indeed displays the predicted activity, namely O-ureidoserine racemization.
The enzyme is proposed to be an epimerase based on sequence similarity to a known enzyme DapF (diaminopimelate epimerase), and the authors show that DscC indeed displays the predicted activity, namely O-ureidoserine racemization.
The determination of the 3D stucture of the enzyme is underway.
‘Comparison of the enzymatic residues that are responsible for recognition of the non-reacting distal site of the enzyme could lead to the ability to rationally design enzymes that can induce epimerization of other amino acids’, conclude the authors.
Read more and access the full article here – FREE to download for a period of four weeks!
Characterization of DcsC, a PLP-independent racemase involved in the biosynthesis of D-cycloserine
David Dietrich, Marco J. van Belkum and John C. Vederas
Org. Biomol. Chem., 2012, 10, 2248-2254
DOI: 10.1039/C2OB06864H,










