The 1st Organic & Biomolecular Chemistry International symposium is underway. This event, visiting three prominent organic chemistry departments in China, brings together speakers from China, Europe and North America.
First stop for the symposium was Shanghai Institute of Organic Chemistry, and day was started with an introduction from SIOC Director Professor Kuling Ding. The opening lecture was given by Professor Guo-Qiang Lin, who gave an overview of his work on the synthesis of chiral dienes, useful ligands for transition metal catalysis. Following that OBC Chair Professor Jeffrey Bode (ETH Zurich, Switzerland) presented developments from his group on amide-forming reactions. After the break Professor Dawei Ma (SIOC) showed some of his recent work in the synthesis of the bioactive alkaloids Berbamine and the Communesins.
After lunch Professor Dirk Trauner (LMU Munich, Germany) gave an energetic overview of a number of natural product syntheses, probing reactions beyond the limits of biomimetic synthesis. Next up was Professor Xuhong Qian (East China University of Science and Technology, Shanghai), who gave an overview of his development of dyes for fluorescent sensing and anticancer applications. After coffee Professor Andrei Yudin (University of Toronto) described a number of areas where his group has been using amphoteric molecules, and the day was rounded off by Professor Yong Tang (SIOC) and his work with pendant-bisoxazoline ligands.
Many thanks to all the speakers, chairs and attendees for making this a successful start to the OBC Symposium. Particular thanks go to Professor Shuli You and his colleagues at SIOC for the local organisation of the meeting.
Next stop, Lanzhou….

















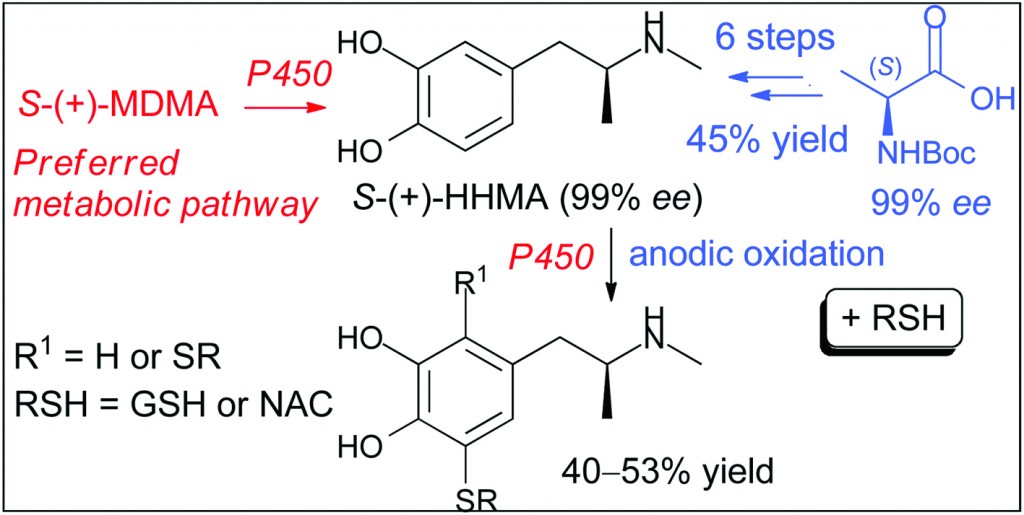 MDMA, also known as “ecstasy”, is a psychoactive drug with selective neurotoxic potential toward brain serotonin neurons. Despite intensive research, the precise mechanism by which MDMA selectively damages brain neurons in most species remains unknown. One hypothesis is that MDMA neurotoxicity may at least partially be a consequence of its metabolism. In particular, O-demethylenated MDMA metabolites such as N-methyl-α-methyldopamine (HHMA) have been postulated to serve as precursors for toxic catechol–thioether conjugates.
MDMA, also known as “ecstasy”, is a psychoactive drug with selective neurotoxic potential toward brain serotonin neurons. Despite intensive research, the precise mechanism by which MDMA selectively damages brain neurons in most species remains unknown. One hypothesis is that MDMA neurotoxicity may at least partially be a consequence of its metabolism. In particular, O-demethylenated MDMA metabolites such as N-methyl-α-methyldopamine (HHMA) have been postulated to serve as precursors for toxic catechol–thioether conjugates.