The Organic & Biomolecular Chemistry web theme issue focussing on the exciting area of foldamer chemistry is now available for you to access, with 21 articles for you to read, 4 of which have so far appeared on the covers of issues.
Here are some of the popular articles that others have been reading:
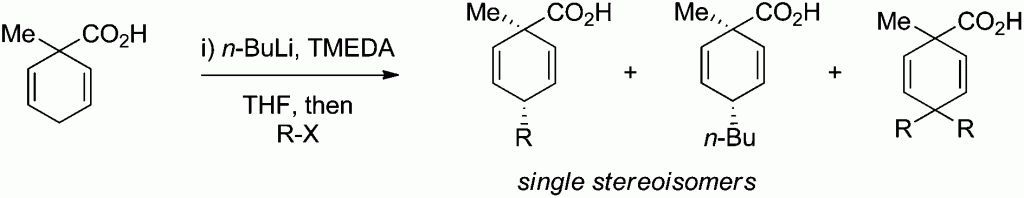
Regio- and diastereoselective fluorination of alicyclic β-amino acids
Loránd Kiss, Enikő Forró, Santos Fustero and Ferenc Fülöp
Org. Biomol. Chem., 2011, 9, 6528-6534
DOI: 10.1039/C1OB05648D
Conformational stability of collagen triple helices functionalized in the Yaa position by click chemistry
Roman S. Erdmann and Helma Wennemers
Org. Biomol. Chem., 2012, 10, 1982-1986
DOI: 10.1039/C2OB06720J
Stereoselective preparation of β,γ-methano-GABA derivatives
David J. Aitken, Ludovic Drouin, Sarah Goretta, Régis Guillot, Jean Ollivier and Marco Spiga
Org. Biomol. Chem., 2011, 9, 7517-7524
DOI: 10.1039/C1OB06095C
Design and synthesis of trans-3-aminopyran-2-carboxylic acid (APyC) and α/β-peptides with 9/11-helix
Gangavaram V. M. Sharma, Kodeti Srinivas Reddy, Shaik Jeelani Basha, Kondreddi Ravinder Reddy and Akella V. S. Sarma
Org. Biomol. Chem., 2011, 9, 8102-8111
DOI: 10.1039/C1OB06279D
To read the rest of this great web themed issue… CLICK HERE
| What do you think if this area of chemistry? Do you have a favourite article from the collection? Let us know your thoughts by leaving a comment below. |















![GA[5]](https://blogs.rsc.org/ob/files/2012/05/GA5-300x141.gif)





![GA[6]](https://blogs.rsc.org/ob/files/2012/04/GA6-300x103.gif)