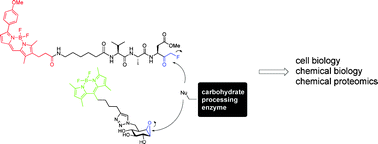
Glycoconjugates play a major role in a number of biological processes, for example modulating cellular interactions, and chemical tools have been instrumental in understanding these aspects of the glycosciences.
In this OBC Perspective Herman Overkleeft and co-authors provide an overview of the enzymes involved in the degradation of glycoconjugates, and the activity-based probes that have been used to study these enzymes, along with the advantages and disadvantages of these probes. Addressing all facets of carbohydrate enzymology, the authors identify a number of key areas for future development in the field.
Are you a glycoscientist or a bioorganic chemist? Then this OBC Perspective is for you! Download the article to read the full details:
Irreversible inhibitors and activity-based probes as research tools in chemical glycobiology
Martin D. Witte, Gijsbert A. van der Marel, Johannes M. F. G. Aerts and Herman S. Overkleeft
Org. Biomol. Chem., 2011, Advance Article
DOI: 10.1039/C1OB05531C, Perspective











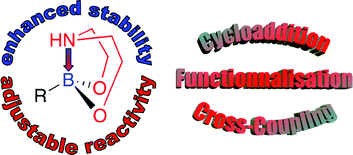 This Emerging Area article from Hélène Bonin and Emmanuel Gras (CNRS) discusses the recent developments in dioxazaborocane chemistry that the authors believe may make it a viable fluoride-free alternative to organotrifluoroborate salts.
This Emerging Area article from Hélène Bonin and Emmanuel Gras (CNRS) discusses the recent developments in dioxazaborocane chemistry that the authors believe may make it a viable fluoride-free alternative to organotrifluoroborate salts.
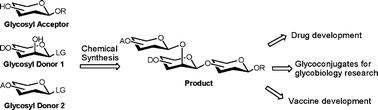 Carbohydrates are the most diverse and one of the most important classes of biomolecules in nature. However, it is still difficult to find carbohydrate-based drugs in the market nowadays. The high polarity of this kind of drugs represents an issue for the pharmacological properties. Therefore, understanding and being able to modify these properties would help with the development of more ‘drug-like’ carbohydrate-based drugs.
Carbohydrates are the most diverse and one of the most important classes of biomolecules in nature. However, it is still difficult to find carbohydrate-based drugs in the market nowadays. The high polarity of this kind of drugs represents an issue for the pharmacological properties. Therefore, understanding and being able to modify these properties would help with the development of more ‘drug-like’ carbohydrate-based drugs.