Achieving sensitive signal detection in fluorescence-based assays often involves chemically pre-amplifying the signal to detectable levels. There are currently several methods of achieving amplification, but here Andrei Kutateladze and colleagues at the University of Denver come up with a concept different from previous methods based on photoamplified fluorescence quenching.

- General concept for the amplified unmasking of benzophenone resulting in fluorescence quenching
They are able to detect molecular recognition events in the biotin-avidin system through the autocatalytic photo-unmasking of benzophenone – which acts as both the amplification chain carrier and the quencher for the reporter fluorophore – achieving attomolar detection limits with a relatively cheap CCD camera. They even demonstrate the possibility of imaging the binding assay with an ordinary mobile phone camera, showing that sensitive analysis can be carried out when expensive state-of-the-art equipment is not available.
To read more, download the article – it’s free to access for the next four weeks:
Photochemically amplified detection of molecular recognition events: an ultra-sensitive fluorescence turn-off binding assay
Tiffany P. Gustafson, Greg A. Metzel and Andrei G. Kutateladze
Org. Biomol. Chem., 2011, Advance Article
DOI: 10.1039/C1OB05289F











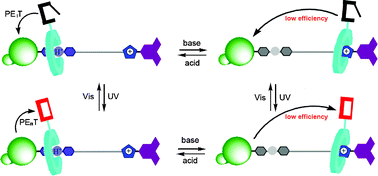 In this HOT paper, Da-Hui Qu, He Tian and their research group at East China University of Science & Technology in Shanghai, synthesise a multi-state [2]rotaxane based on a crown ether. They introduce a dithienylethene (DTE) photochromic functional group that can be switched by pH, light or the combination of pH and light. This photochromic unit is responsible of the multi-mode alteration of intercomponent interactions such as energy transfer, electron transfer and charge transfer interactions.
In this HOT paper, Da-Hui Qu, He Tian and their research group at East China University of Science & Technology in Shanghai, synthesise a multi-state [2]rotaxane based on a crown ether. They introduce a dithienylethene (DTE) photochromic functional group that can be switched by pH, light or the combination of pH and light. This photochromic unit is responsible of the multi-mode alteration of intercomponent interactions such as energy transfer, electron transfer and charge transfer interactions.