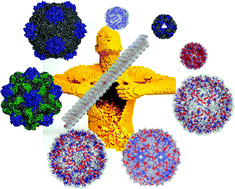
Nature has designed many biogenic systems with exquisite symmetries and complexities on the nanometer scale, such as viruses, ferritins and enzyme complexes. These structures have acted as building blocks on which researchers have been able to develop bionanoparticles with a wide variety of applications including biosensors, electronic nanodevices, drug delivery agents and vaccine carriers, amongst others.
In this OBC Perspective Qian Wang and colleagues highlight some of the recent progress in the chemical modification and molecular engineering of these bionanoparticles with the aim of sparking new discussions and inspiring the development of many new materials in the future.
Interested? Then why not read this comprehensive review now. It is free to download for the next four weeks!
Altering the landscape of viruses and bionanoparticles
L. Andrew Lee, Huong Giang Nguyen and Qian Wang
Org. Biomol. Chem., 2011, DOI: 10.1039/C1OB05700F












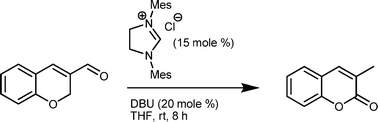

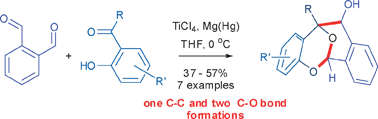 An expeditious one-step entry to the tetracyclic core of integrastatins
An expeditious one-step entry to the tetracyclic core of integrastatins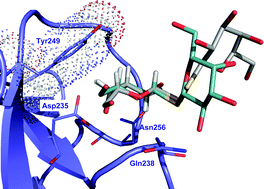 S-glycosides are resistant to hydrolytic cleavage, which gives them a distinct pharmacological advantage over O-glycosides as ligands for lectins, but inherently present different biological activity. This HOT article evaluates the ability of dithiodigalactoside (DTDG) – which was identified through a dynamic combinatorial library approach in a previous study – to protect human cells from toxin binding.
S-glycosides are resistant to hydrolytic cleavage, which gives them a distinct pharmacological advantage over O-glycosides as ligands for lectins, but inherently present different biological activity. This HOT article evaluates the ability of dithiodigalactoside (DTDG) – which was identified through a dynamic combinatorial library approach in a previous study – to protect human cells from toxin binding.