The cool images on this week’s cover are from Giuseppe Resnati, NFMLab, and James C. Anderson, UCL.
 Resnati and colleagues have conducted an NMR study of phosphate oxoanion binding of a 2-iodo-imidazolium receptor with charge assisted halogen bonding, finding a surprisingly strong binding constant for phosphate anions, their work is highlighted on the outside front cover.
Resnati and colleagues have conducted an NMR study of phosphate oxoanion binding of a 2-iodo-imidazolium receptor with charge assisted halogen bonding, finding a surprisingly strong binding constant for phosphate anions, their work is highlighted on the outside front cover.
2-Iodo-imidazolium receptor binds oxoanions via charge-assisted halogen bonding
Massimo Cametti, Kari Raatikainen, Pierangelo Metrangolo, Tullio Pilati, Giancarlo Terraneo and Giuseppe Resnati
DOI: 10.1039/C1OB06524F
 On a completely different topic, Anderson and Moreno have been using CO2 to produce ureas at ambient conditions, using 12- and 14- electron titanium imido complexes:
On a completely different topic, Anderson and Moreno have been using CO2 to produce ureas at ambient conditions, using 12- and 14- electron titanium imido complexes:
Synthesis of ureas from titanium imido complexes using CO2 as a C-1 reagent at ambient temperature and pressure
James C. Anderson and Rafael Bou Moreno
DOI: 10.1039/C1OB06576A
As well as these the issue also contains a review from Tim Donohoe on olefin cross-metathesis for heterocycle synthesis and plenty of other hot articles, take a look…
Hot articles in this issue:
Some cyclization reactions of 1,3-diphenylbenzo[e][1,2,4]triazin-7(1H)-one: preparation and computational analysis of non symmetrical zwitterionic biscyanines
Theodosia A. Ioannou, Panayiotis A. Koutentis, Harry Krassos, Georgia Loizou and Daniele Lo Re
DOI: 10.1039/C1OB06622F
Oxidative Prins and Prins/Friedel–Crafts cyclizations for the stereoselective synthesis of dioxabicycles and hexahydro-1H-benzo[f]isochromenes via the benzylic C–H activation
B. V. Subba Reddy, Prashant Borkar, J. S. Yadav, P. Purushotham Reddy, A. C. Kunwar, B. Sridhar and René Grée
DOI: 10.1039/C1OB06489D
Chemoenzymatic synthesis of a mixed phosphine–phosphine oxide catalyst and its application to asymmetric allylation of aldehydes and hydrogenation of alkenes
Derek R. Boyd, Mark Bell, Katherine S. Dunne, Brian Kelly, Paul J. Stevenson, John F. Malone and Christopher C. R. Allen
DOI: 10.1039/C1OB06599H
James C. Anderson and Rafael Bou Moreno
Org. Biomol. Chem., 2012, 10, 1334-1338
DOI: 10.1039/C1OB06576A
Certain 12- and 14- electron titanium imido complexes











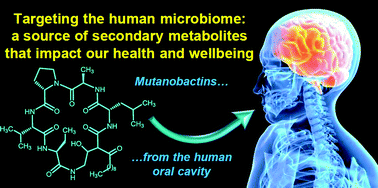 A bacteria present in the mouth – Streptococcus mutans – has been shown to generate the metabolite mutanobactin A by scientists from the US. They found that the metabolite inhibits biofilms formed by the fungal oral pathogen Candida albicans, which causes yeast infections.
A bacteria present in the mouth – Streptococcus mutans – has been shown to generate the metabolite mutanobactin A by scientists from the US. They found that the metabolite inhibits biofilms formed by the fungal oral pathogen Candida albicans, which causes yeast infections. On the cover of
On the cover of 
 Resnati and colleagues have conducted an NMR study of phosphate oxoanion binding of a 2-iodo-imidazolium receptor with charge assisted halogen bonding, finding a surprisingly strong binding constant for phosphate anions, their work is highlighted on the outside front cover.
Resnati and colleagues have conducted an NMR study of phosphate oxoanion binding of a 2-iodo-imidazolium receptor with charge assisted halogen bonding, finding a surprisingly strong binding constant for phosphate anions, their work is highlighted on the outside front cover. On a completely different topic, Anderson and Moreno have been using CO2 to produce ureas at ambient conditions, using 12- and 14- electron titanium imido complexes:
On a completely different topic, Anderson and Moreno have been using CO2 to produce ureas at ambient conditions, using 12- and 14- electron titanium imido complexes:
 On the outside front cover of this week’s issue is a
On the outside front cover of this week’s issue is a  The inside front cover is work from Jonathan Guimond-Tremblay, Université Laval, et al. on the synthesis of three monofluorinated dimyristoylphosphatidylcholine derivatives for use as probes in 19F NMR studies to study the effects of drugs and peptides on model membranes.
The inside front cover is work from Jonathan Guimond-Tremblay, Université Laval, et al. on the synthesis of three monofluorinated dimyristoylphosphatidylcholine derivatives for use as probes in 19F NMR studies to study the effects of drugs and peptides on model membranes.