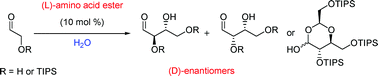
A conundrum for anyone studying the prebiotic world is how chirality first occurred, and why D-sugars are the natural isomers, whereas natural amino acids have the L-configuration. In this hot paper Paul Clarke and Christopher Hayes from the Universities of York and Nottingham have shown how carbohydrates could have formed in prebiotic conditions by producing (D)-erythrose and (D)-threose sugars with (L)-amino acid catalysts.
Having first used proline esters to catalyse the aldol reaction of TIPS-protected glycolaldehyde in water with good yields and moderate diastereo- and enantioselectivity, the group hypothesised that other proteinogenic amino acids (i.e. amino acids found in proteins) could also catalyse this reaction. Using N-methyl derivatives of the amino acid esters of (L)-alanine, (L)-leucine and (L)-valine they were able to produce the expected sugars, but were surprised to see that the (D)-enantiomer was the major product in all reactions. “This offers one potential explanation to account for the relationship between (L)-amino acids and (D)-sugars in nature” concludes Clarke.
This remarkable find has obviously generated a lot of interest in both scientific and the wider press: see articles in The Daily Mail, The Huffington Post, on Science Daily or the press release from University of York.
Asymmetric organocatalytic formation of protected and unprotected tetroses under potentially prebiotic conditions
Laurence Burroughs, Paul A. Clarke, Henrietta Forinto, James A. R. Gilks, Christopher J. Hayes, Matthew E. Vale , William Wade and Myriam Zbytniewski
DOI: 10.1039/C1OB06798B
This article is also part of our joint web theme issue with ChemComm on Organocatalysis and as with all our hot articles will be free to access for 4 weeks.