| In May 2014, Ian Paterson celebrates his sixtieth birthday. Eighty students have completed PhDs under his guidance. To mark this milestone, the RSC are collating the papers he has published in RSC journals and making them available to everybody. Ian was born in Dundee, and studied at St Andrew’s University. Two papers were published in the Journal of the Chemical Society as a result of his undergraduate research. He then moved to the University of Cambridge to work with Ian Fleming and to contribute further to RSC publications. A postdoctoral fellowship at Columbia University followed, working with Gilbert Stork, who had published fewer papers with the RSC than Ian. Ian then returned to the UK, first to UCL and then to Cambridge, where he has been for the last three decades.He has developed new methods for stereoselective transformations, particularly the boron-mediated aldol reaction, and used these in the syntheses of larger and larger molecules with increasing stereochemical complexity. The first report of his approach to discodermolide appeared in Chem Comm (1993) and, in due course, the synthesis was scaled up so that it could be used for clinical development. Studies on spongistatin, spirastrellolide, dictyostatin and other complex natural products have all been reported in RSC journals, as well as investigations of hybrids and semi-synthetic analogues. Ian has been elected to fellowships both of the Royal Society and the Royal Society of Edinburgh, and has won many RSC prizes including the Meldola Prize (1983), Hickinbottom Fellowship (1989), Bader Award (1996), Synthetic Organic Chemistry Award (2001), and the Tilden Prize (2009).Professor Jonathan Goodman University of Cambridge, UK |
All articles are free to access until 13th June 2014
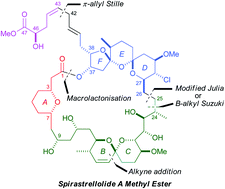
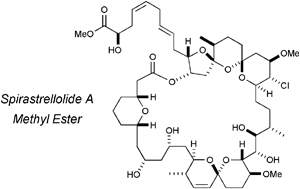
The stereocontrolled total synthesis of spirastrellolide A methyl ester. Fragment coupling studies and completion of the synthesis
Ian Paterson, Edward A. Anderson, Stephen M. Dalby, Jong Ho Lim and Philip Maltas
Org. Biomol. Chem., 2012,10, 5873-5886, DOI: 10.1039/C2OB25101A
The stereocontrolled total synthesis of spirastrellolide A methyl ester. Expedient construction of the key fragments
Ian Paterson, Edward A. Anderson, Stephen M. Dalby, Jong Ho Lim, Philip Maltas, Olivier Loiseleur, Julien Genovino and Christian Moessner
Org. Biomol. Chem., 2012,10, 5861-5872, DOI: 10.1039/C2OB25100K
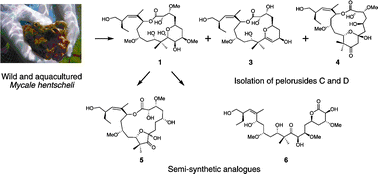
Structure–activity studies of the pelorusides: new congeners and semi-synthetic analogues
A. Jonathan Singh, Mina Razzak, Paul Teesdale-Spittle, Thomas N. Gaitanos, Anja Wilmes, Ian Paterson, Jonathan M. Goodman, John H. Miller and Peter T. Northcote
Org. Biomol. Chem., 2011,9, 4456-4466, DOI: 10.1039/C0OB01127D
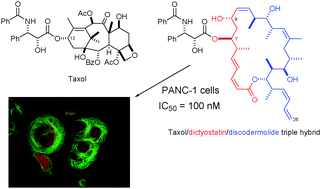
Total synthesis of a library of designed hybrids of the microtubule-stabilising anticancer agents taxol, discodermolide and dictyostatin
Ian Paterson, Guy J. Naylor, Takeshi Fujita, Esther Guzmán and Amy E. Wright
Chem. Commun., 2010,46, 261-263, DOI: 10.1039/B921237J
Synthesis and stereochemical determination of the spirastrellolides
Ian Paterson and Stephen M. Dalby
Nat. Prod. Rep., 2009,26, 865-873, DOI: 10.1039/B906991G
Total synthesis of (-)-spirangien A and its methylester
Ian Paterson, Alison D. Findlay and Christian Noti
Chem. Commun., 2008, 6408-6410, DOI: 10.1039/B816229H
Total synthesis of the marine macrolide (+)-neopeltolide
Ian Paterson and Natalie A. Miller
Chem. Commun., 2008, 4708-4710, DOI: 10.1039/B812914B
Total synthesis of a potent hybrid of the anticancer natural products dictyostatin and discodermolide
Ian Paterson, Guy J. Naylor and Amy E. Wright
Chem. Commun., 2008, 4628-4630, DOI: 10.1039/B811575C
Development of practical syntheses of the marine anticancer agents discodermolide and dictyostatin
Gordon J. Florence, Nicola M. Gardner and Ian Paterson
Nat. Prod. Rep., 2008,25, 342-375, DOI: 10.1039/B705661N
Synthesis of two diastereomeric C1–C22 fragments of spirastrellolide A
Ian Paterson, Edward A. Anderson, Stephen M. Dalby, Julien Genovino, Jong Ho Lim and Christian Moessner
Chem. Commun., 2007, 1852-1854, DOI: 10.1039/B700827A
Design, synthesis and biological evaluation of a macrocyclic discodermolide/dictyostatin hybrid
Ian Paterson and Nicola M. Gardner
Chem. Commun., 2007, 49-51, DOI: 10.1039/B615122A
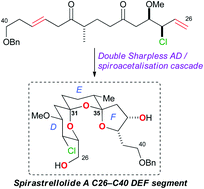
Synthesis of the DEF-bis-spiroacetal of spirastrellolide A exploiting a double asymmetric dihydroxylation/spiroacetalisation strategy
Ian Paterson, Edward A. Anderson, Stephen M. Dalby, Jong Ho Lim, Philip Maltas and Christian Moessner
Chem. Commun., 2006, 4186-4188, DOI: 10.1039/B612697A
Towards the combinatorial synthesis of spongistatin fragment libraries by using asymmetric aldol reactions on solid support
Ian Paterson, Dirk Gottschling and Dirk Menche
Chem. Commun., 2005, 3568-3570, DOI: 10.1039/B505746A
The stereocontrolled total synthesis of altohyrtin A/spongistatin 1: the CD-spiroacetal segment
Ian Paterson, Mark J. Coster, David Y.-K. Chen, Karl R. Gibson and Debra J. Wallace
Org. Biomol. Chem., 2005,3, 2410-2419, DOI: 10.1039/B504148A
The stereocontrolled total synthesis of altohyrtin A/spongistatin 1: the AB-spiroacetal segment
Ian Paterson, Mark J. Coster, David Y.-K. Chen, Renata M. Oballa, Debra J. Wallace and Roger D. Norcross
Org. Biomol. Chem., 2005,3, 2399-2409, DOI: 10.1039/B504146E
The stereocontrolled total synthesis of altohyrtin A/spongistatin 1: fragment couplings, completion of the synthesis, analogue generation and biological evaluation
Ian Paterson, David Y.-K. Chen, Mark J. Coster, José L. Aceña, Jordi Bach and Debra J. Wallace
Org. Biomol. Chem., 2005,3, 2431-2440, DOI: 10.1039/B504151A
The stereocontrolled total synthesis of altohyrtin A/spongistatin 1: the southern hemisphere EF segment
Ian Paterson, Mark J. Coster, David Y.-K. Chen, José L. Aceña, Jordi Bach, Linda E. Keown and Thomas Trieselmann
Org. Biomol. Chem., 2005,3, 2420-2430, DOI: 10.1039/B504149J
Phorboxazole B synthetic studies: construction of C(1–32) and C(33–46) subtargets
Ian Paterson, Alan Steven and Chris A. Luckhurst
Org. Biomol. Chem., 2004,2, 3026-3038, DOI: 10.1039/B407240E
Stereochemical determination of dictyostatin, a novel microtubule-stabilising macrolide from the marine sponge Corallistidae sp.
Ian Paterson, Robert Britton, Oscar Delgado and Amy E. Wright
Chem. Commun., 2004, 632-633, DOI: 10.1039/B316390C
Synthesis and biological evaluation of spongistatin/altohyrtin analogues: E-ring dehydration and C46 side-chain truncation
Ian Paterson, Jose L. Aceña, Jordi Bach, David Y.-K. Chen and Mark J. Coster
Chem. Commun., 2003, 462-463, DOI: 10.1039/B212651F
Laboratory emulation of polyketide biosynthesis: an iterative, aldol-based, synthetic entry to polyketide libraries using (R)- and (S )-1-(benzyloxy)-2-methylpentan-3-one, and conformational aspects of extended polypropionates
Ian Paterson and Jeremy P. Scott
J. Chem. Soc., Perkin Trans. 1, 1999, 1003-1014, DOI: 10.1039/A809818B
Recent developments in asymmetric aldol methodology
Alison S. Franklin and Ian Paterson
Contemp. Org. Synth., 1994,1, 317-338, DOI: 10.1039/CO9940100317
Studies towards the total synthesis of the marine-derived immunosuppressant discodermolide; asymmetric synthesis of a C1–C8d-lactone subunit
Ian Paterson and Stephen P. Wren
J. Chem. Soc., Chem. Commun., 1993, 1790-1792, DOI: 10.1039/C39930001790
The formation of allyl sulphides by phenylthio-migration: control by silicon
Ian Fleming, Ian Paterson and Andrew Pearce
J. Chem. Soc., Perkin Trans. 1, 1981, 256-262, DOI: 10.1039/P19810000256
Free radical addition to olefins. Part 23. Kinetics of the addition of chloroiodomethane to ethylene and vinyl fluoride
Ian Paterson, John M. Tedder and John C. Walton
J. Chem. Soc., Perkin Trans. 2, 1978, 884-887, DOI: 10.1039/P29780000884
Homosolvolysis
Hamish Low, Ian Paterson, John M. Tedder and John Walton
J. Chem. Soc., Chem. Commun., 1977, 171-172, DOI: 10.1039/C39770000171