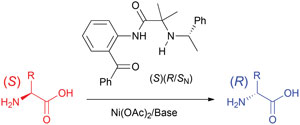
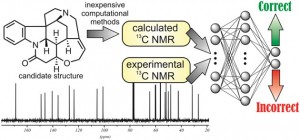
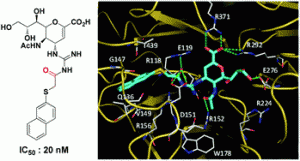
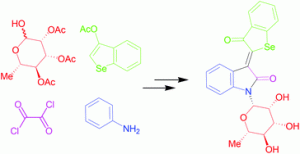
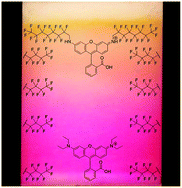
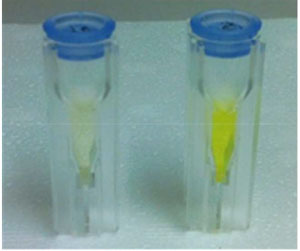
It is next to impossible to achieve good mixing with traditional magnetic stir bars in the cylindrical vessels used in microwave synthesis. So scientists in Austria have designed a new stir bar.
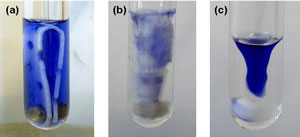 Microwave synthesis is becoming more popular thanks to the dramatically reduced reactions times and improved yields it offers. However, as the majority of these reactions involve a small narrow reaction vessel, traditional horizontal stir bars often result in inefficient mixing which can cause temperature gradients to develop and reduce product yields.
Microwave synthesis is becoming more popular thanks to the dramatically reduced reactions times and improved yields it offers. However, as the majority of these reactions involve a small narrow reaction vessel, traditional horizontal stir bars often result in inefficient mixing which can cause temperature gradients to develop and reduce product yields.
Magnetic stirring usually involves a polymer coated AlNiCo or ferrite magnet and relies on the establishment of a liquid flow induced by centrifugal forces. These flow patterns involve a downward liquid motion towards the centre and an upward motion at the vessel wall. However, the strength of the magnet decreases rapidly over a period of months when subjected to the high temperatures of microwave synthesis and the limited size of the vessel restricts the establishment of flow patterns.
‘We noticed that in some instances the agitation of the reaction mixture using a standard magnetic stir bar was very poor. Sometimes the stirrer was not moving at all,’ says Oliver Kappe whose team at the University of Graz used a camera to look at a microwave system before designing the new stir bar.
Choice of magnetic material and shape were thought to be key to the success of the new stir bar. Vertical blade extensions were added to a more robust Sm2Co17 rare earth magnet. This not only extends the life of the stir bar but also significantly enlarges the cross-sectional area of the stirrer. A gap in the centre of the bar guarantees backflow which enforces flow patterns and extended blades mean that even the upper part of the system receives efficient mixing with the incorporation of additional holes and cut-outs inducing additional turbulence.
Read the full story in Chemistry World
Design and evaluation of improved magnetic stir bars for single-mode microwave reactor
David Obermayer, Markus Damm and C. Oliver Kappe
Org. Biomol. Chem., 2013, Accepted Manuscript
DOI: 10.1039/C3OB40790J