We are delighted to share with you a series of collections of recent books, themed issues and articles on the topic of water. These four collections – one per month – demonstrate different aspects of water: its chemistry, its wide use in reactions and as a solvent, its relationship with energy and sustainability, as well as with human health and the environment.
Here, in our second collection, we have assembled some of the ground-breaking research and transformative reviews related to the chemistry in water – highlighting the importance and versatility of water as a medium in chemistry – from across our journals.
Professor Rafael Luque, winner of the RSC 2013 Environment, Sustainability and Energy Division Early Career Award, welcomes this timely and topical collection. “For a more sustainable future, we need to develop benign chemical protocols,” he notes. “Conducting chemical processes in water is a significant step towards this goal. This high profile collection includes articles from leading scientists in the field with varying topics – from materials synthesis to organocatalysis, synthetic organic chemistry and heterogeneous catalysis – that we hope can provide a starting point for young researchers, as well as key references to stimulate further research in the field.”
“This year, as the IPCC prepares to release the final contributions to their Fifth Assessment Report on climate change, it is timely to consider the role of chemistry in addressing global challenges, such as food, water, raw materials and energy,” remarks Professor Lesley Yellowlees, President of the Royal Society of Chemistry. “This collection from our journals shares the latest research from scientists around the world, aiming to tackle these challenges. Featuring original research and commentary by leaders in the field, we hope that you will find this high-quality collection engaging, inspirational and informative.”
You can read all of these articles for free until 21 April 2014! We truly hope you enjoy this collection.
We have already published an article collection on the chemistry of water. In the next couple of months, we will be publishing collections on water in energy, health and the environment.
Did you know that the RSC has put together a webpage on Water, which brings together information on activities for scientists, policymakers, educators and young people? Take a look today…
Reviews and Perspectives
In water, on water, and by water: mimicking nature’s aldolases with organocatalysis and water
Nobuyuki Mase and Carlos F. Barbas, III
Org. Biomol. Chem., 2010,8, 4043-4050
DOI: 10.1039/C004970K, Perspective
Benign by design: catalyst-free in-water, on-water green chemical methodologies in organic synthesis
Manoj B. Gawande, Vasco D. B. Bonifácio, Rafael Luque, Paula S. Branco and Rajender S. Varma
Chem. Soc. Rev., 2013,42, 5522-5551
DOI: 10.1039/C3CS60025D, Review Article
Olefin metathesis in aqueous media
Jasmine Tomasek and Jürgen Schatz
Green. Chem., 2013,15, 2317-2338
DOI: 10.1039/C3GC41042K, Critical Review
Green chemistry oriented organic synthesis in water
Marc-Olivier Simon and Chao-Jun Li
Chem. Soc. Rev., 2012,41, 1415-1427
DOI: 10.1039/C1CS15222J, Tutorial Review
From themed collection Green Chemistry
sp2 C–H bond activation in water and catalytic cross-coupling reactions
Bin Li and Pierre H. Dixneuf
Chem. Soc. Rev., 2013,42, 5744-5767
DOI: 10.1039/C3CS60020C, Review Article
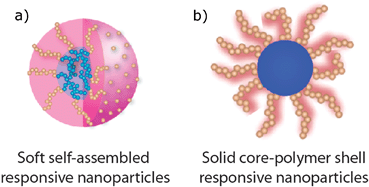
Stimulus responsive core-shell nanoparticles: synthesis and applications of polymer based aqueous systems
Olivier J. Cayre, Nelly Chagneux and Simon Biggs
Soft Matter, 2011,7, 2211-2234
DOI: 10.1039/C0SM01072C, Review Article
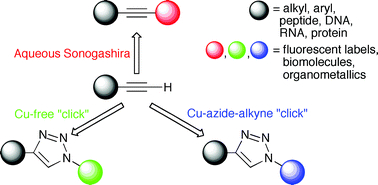
Alkynes as an eco-compatible “on-call” functionality orthogonal to biological conditions in water
Nick Uhlig and Chao-Jun Li
Chem. Sci., 2011,2, 1241-1249
DOI: 10.1039/C1SC00164G, Minireview
Anion binding in water at lanthanide centres: from structure and selectivity to signalling and sensing
Stephen J. Butler and David Parker
Chem. Soc. Rev., 2013,42, 1652-1666
DOI: 10.1039/C2CS35144G, Tutorial Review
From themed collection Alfred Werner Nobel Prize 100 year celebration
Prebiotic chemistry in eutectic solutions at the water–ice matrix
César Menor-Salván and Margarita R. Marín-Yaseli
Chem. Soc. Rev., 2012,41, 5404-5415
DOI: 10.1039/C2CS35060B, Tutorial Review
From themed collection Prebiotic Chemistry
Original research articles
An in-water, on-water domino process for synthesis
Philip Norcott, Calan Spielman and Christopher S. P. McErlean
Green. Chem., 2012,14, 605-609
DOI: 10.1039/C2GC16259H, Communication
Highly efficient iron(0) nanoparticle-catalyzed hydrogenation in water in flow
Reuben Hudson, Go Hamasaka, Takao Osako, Yoichi M. A. Yamada, Chao-Jun Li, Yasuhiro Uozumi and Audrey Moores
Green. Chem., 2013,15, 2141-2148
DOI: 10.1039/C3GC40789F, Paper
Water as an additive to enhance the ring opening of naphthalene
Qian Wang, Honglei Fan, Suxiang Wu, Zhaofu Zhang, Peng Zhang and Buxing Han
Green. Chem., 2012,14, 1152-1158
DOI: 10.1039/C2GC16554F, Paper
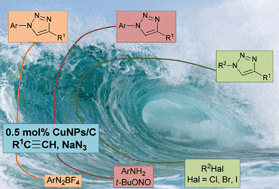
Click chemistry from organic halides, diazonium salts and anilines in water catalysed by copper nanoparticles on activated carbon
Francisco Alonso, Yanina Moglie, Gabriel Radivoy and Miguel Yus
Org. Biomol. Chem., 2011,9, 6385-6395
DOI: 10.1039/C1OB05735A, Paper
An organocatalytic highly efficient approach to the direct synthesis of substituted carbazoles in water
Pradeep Kumar Jaiswal, Soumen Biswas, Shivendra Singh and Sampak Samanta
Org. Biomol. Chem., 2013,11, 8410-8418
DOI: 10.1039/C3OB42034E, Paper
Near-critical water, a cleaner solvent for the synthesis of a metal–organic framework
Ilich A. Ibarra, Peter A. Bayliss, Eduardo Pérez, Sihai Yang, Alexander J. Blake, Harriott Nowell, David R. Allan, Martyn Poliakoff and Martin Schröder Green Chem., 2012,14, 117-122
DOI: 10.1039/C1GC15726D, Paper
Stille couplings in water at room temperature
Guo-ping Lu, Chun Cai and Bruce H. Lipshutz
Green Chem., 2013,15, 105-109
DOI: 10.1039/C2GC36042J, Paper
DNA-based asymmetric organometallic catalysis in water
Jens Oelerich and Gerard Roelfes
Chem. Sci., 2013,4, 2013-2017
DOI: 10.1039/C3SC00100H, Edge Article
Pyridinium-based tripodal chemosensor in visual sensing of AMP in water by indicator displacement assay (IDA)
Kumaresh Ghosh, Sk Sarfaraj Ali, Avik Ranjan Sarkar, Asmita Samadder, Anisur Rahman Khuda-Bukhsh, Ioannis D. Petsalakis and Giannoula Theodorakopoulos
Org. Biomol. Chem. , 2013, 11, 5666-5672
DOI: 10.1039/C3OB40833G, Paper
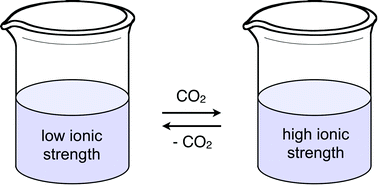
Design, synthesis, and solution behaviour of small polyamines as switchable water additives
Sean M. Mercer, Tobias Robert, Daniel V. Dixon, Chien-Shun Chen, Zahra Ghoshouni, Jitendra R. Harjani, Soran Jahangiri, Gilles H. Peslherbe and Philip G. Jessop
Green Chem. , 2012,14, 832-839
DOI: 10.1039/C2GC16240G, Paper
In situ generation of bioreducible and acid labile nanogels/microgels simply via adding water into the polymerization system
Zhong-Kai Wang, Long-Hai Wang, Jiao-Tong Sun, Li-Fen Han and Chun-Yan Hong
Polym. Chem., 2013, 4, 1694-1699
DOI: 10.1039/C2PY21058D, Paper
Template-directed synthesis of multi-component organic cages in water
Artur R. Stefankiewicz, Mark R. Sambrook and Jeremy K. M. Sanders
Chem. Sci., 2012,3, 2326-2329
DOI: 10.1039/C2SC20347B, Edge Article
Tuning the catalytic activity of L-proline functionalized hydrophobic nanogel particles in water
Annhelen Lu, Dafni Moatsou, Deborah A. Longbottom and Rachel K. O’Reilly
Chem. Sci., 2013,4, 965-969
DOI: 10.1039/C2SC21300A, Edge Article
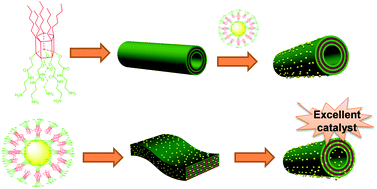
Gold nanoparticles stabilized by an amphiphilic pillar[5]arene: preparation, self-assembly into composite microtubes in water and application in green catalysis
Yong Yao, Min Xue, Zibin Zhang, Mingming Zhang, Yong Wang and Feihe Huang
Chem. Sci., 2013,4, 3667-3672
DOI: 10.1039/C3SC51547H, Edge Article
Water-controlled synthesis and single-crystal structural transformations of a cyanide-bridged W(IV)–Ni(II) molecular wheel complex and 3D networks
Dao-Peng Zhang, Li-Fang Zhang, Guo-Ling Li and Zhong-Hai Ni
Chem. Comm., 2013,49, 9582-9584
DOI: 10.1039/C3CC46063K, Communication
Oxidation of water by a nonhaem diiron(IV) complex via proton-coupled electron transfer
Dong Wang and Lawrence Que
Chem. Comm., 2013,49, 10682-10684
DOI: 10.1039/C3CC46391E, Communication
From themed collection Biological oxidation reactions: mechanisms and design of new catalysts
Enantioselective Friedel–Crafts reactions in water catalyzed by a human telomeric G-quadruplex DNA metalloenzyme
Changhao Wang, Yinghao Li, Guoqing Jia, Yan Liu, Shengmei Lu and Can Li
Chem. Comm., 2012,48, 6232-6234
DOI: 10.1039/C2CC31320K, Communication
Enhanced imine synthesis in water: from surfactant-mediated catalysis to host–guest mechanisms
Kamel Meguellati, Ali Fallah-Araghi, Jean-Christophe Baret, Abdeslam El Harrak, Thomas Mangeat, Carlos M. Marques, Andrew D. Griffiths and Sylvain Ladame
Chem. Comm., 2013,49, 11332-11334
DOI: 10.1039/C3CC46461J, Communication
Open Access
A method for increasing permeability in O2/N2 separation with mixed-matrix membranes made of water-stable MIL-101 and polysulfone
Harold B. Tanh Jeazet, Claudia Staudt and Christoph Janiak
Chem. Comm., 2012,48, 2140-2142
DOI: 10.1039/C2CC16628C, Communication
Cu(OTf)2-catalysed Ritter reaction: efficient synthesis of amides from nitriles and halohydrocarbons in water
Gui-Rong Qu, Yan-Wei Song, Hong-Ying Niu, Hai-Ming Guo and John S. Fossey
RSC Adv., 2012,2, 6161-6163
DOI: 10.1039/C2RA20941A, Communication
Recyclable NaHSO4 catalyzed alkylation of tert-enamides with indoles or amines in water: facile construction of pharmaceutically analogous bis-alkaloid scaffolds
Xue-Qiang Chu, Shun-Yi Wang and Shun-Jun Ji
RSC Adv., 2013,3, 8380-8387
DOI: 10.1039/C3RA40833G, Paper
Efficient microwave-assisted preparation of squaric acid monoamides in water
Carlos López, Manuel Vega, Elena Sanna, Carmen Rotger and Antoni Costa
RSC Adv., 2013,3, 7249-7253
DOI: 10.1039/C3RA41369A, Communication
CuI/TBAB as a novel efficient catalytic system for Heck reaction in water
Yufang Wang, Qichao Yang, Li Yang, Jianxin Shi and Mingjie Zhang
RSC Adv., 2013,3, 21251-21255
DOI: 10.1039/C3RA44819C, Communication
Highly efficient visible-light-induced photocatalytic hydrogenation of nitrobenzene to aniline in water
Weiming Wu, Rui Lin, Lijuan Shen, Ruowen Liang, Rusheng Yuan and Ling Wu
RSC Adv., 2013,3, 10894-10899
DOI: 10.1039/C3RA40935J, Paper
Ruthenium catalysed one-pot synthesis of S-allyl and cinnamyl dithiocarbamates using allyl and cinnamyl acetates in water
Sabir Ahammed, Amit Saha and Brindaban C. Ranu
RSC Adv., 2012,3, 6329-6335
DOI: 10.1039/C2RA20856C, Paper
Water-soluble multi-cage super tetrahedral uranyl peroxide phosphate clusters
Jie Qiu, Jie Ling, Laurent Jouffret, Rebecca Thomas, Jennifer E. S. Szymanowski and Peter C. Burns
Chem. Sci., 2014,5, 303-310
DOI: 10.1039/C3SC52357H, Edge Article
Mechanistic changes observed in heavy water for nitrate reduction reaction on palladium-modified Pt(hkl) electrodes
J. Souza-Garcia, E. A. Ticianelli, V. Climent and J. M. Feliu
Chem. Sci., 2012,3, 3063-3070
DOI: 10.1039/C2SC20490H, Edge Article
Active and reusable Pd(II) organometallic catalyst covalently bonded to mesoporous silica nanospheres for water-medium organic reactions
Wenhan He, Fang Zhang and Hexing Li
Chem. Sci., 2011,2, 961-966
DOI: 10.1039/C0SC00652A, Edge Article
Selective electrocatalytic reduction of carbon dioxide to formate by a water-soluble iridium pincer catalyst
Peng Kang, Thomas J. Meyer and Maurice Brookhart
Chem. Sci., 2013,4, 3497-3502
DOI: 10.1039/C3SC51339D, Edge Article