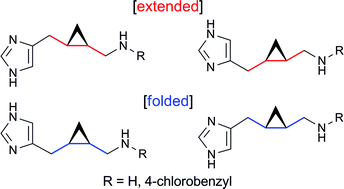
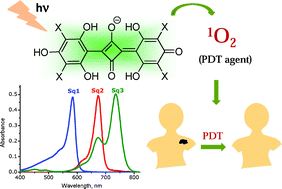
In this OBC Hot Article, Panayiotis Koutentis and co-workers at the University of Cyprus show how 1,3-Diphenylbenzo[e][1,2,4]triazin-7(1H)-one reacts with various bisnucleophiles to give a variety of deeply coloured polyazaacenes, including two zwitterionic analogues.
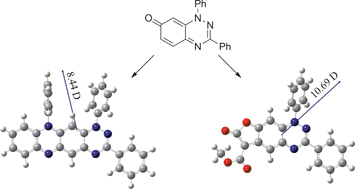
Read the article to find out more about the preparation, mechanistic considerations and photophysical studies of these new heterocyclic ring systems derived from benzo[1,2,4]triazin-7-ones.
FREE to access for a period of 4 weeks!
Some cyclization reactions of 1,3-diphenylbenzo[e][1,2,4]triazin-7(1H)-one: preparation and computational analysis of non symmetrical zwitterionic biscyanines
Theodosia A. Ioannou, Panayiotis A. Koutentis, Harry Krassos, Georgia Loizou and Daniele Lo Re
Org. Biomol. Chem., 2012, Advance Article
DOI: 10.1039/C1OB06622F, Paper












![Rich_med_tcm18-153780[1]](https://blogs.rsc.org/ob/files/2012/01/Rich_med_tcm18-15378013.jpg)
![GA[1]](https://blogs.rsc.org/ob/files/2011/12/GA12.gif)
![GA[1]](https://blogs.rsc.org/ob/files/2011/12/GA11.gif)