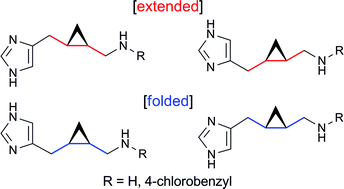
In their quest to develop specific ligands for drug target proteins, Professor Satoshi Shuto and coll. have used a stereochemical diversity-oriented conformational restriction strategy to synthesize potent histamine H3 and/or H4 receptor ligands. Some of these cyclopropane-based analogs of histamine with a chiral cis- or trans-2,3-methanobutane backbone show remarkable antagonistic activity towards H3 and H4.
Interested in Medicinal Chemistry and curious about what conformational restriction strategies can do for you?
Then why not read this article, FREE to access for a period of four weeks
Cyclopropane-based stereochemical diversity-oriented conformational restriction strategy: Histamine H3 and/or H4 receptor ligands with the 2,3-methanobutane backbone
Mizuki Watanabe, Takaaki Kobayashi, Takatsugu Hirokawa, Akira Yoshida, Yoshihiko Ito, Shizuo Yamada, Naoki Orimoto, Yasundo Yamasaki, Mitsuhiro Arisawa and Satoshi Shuto
Org. Biomol. Chem., 2012, Advance Article
DOI: 10.1039/C1OB06496G, Paper










