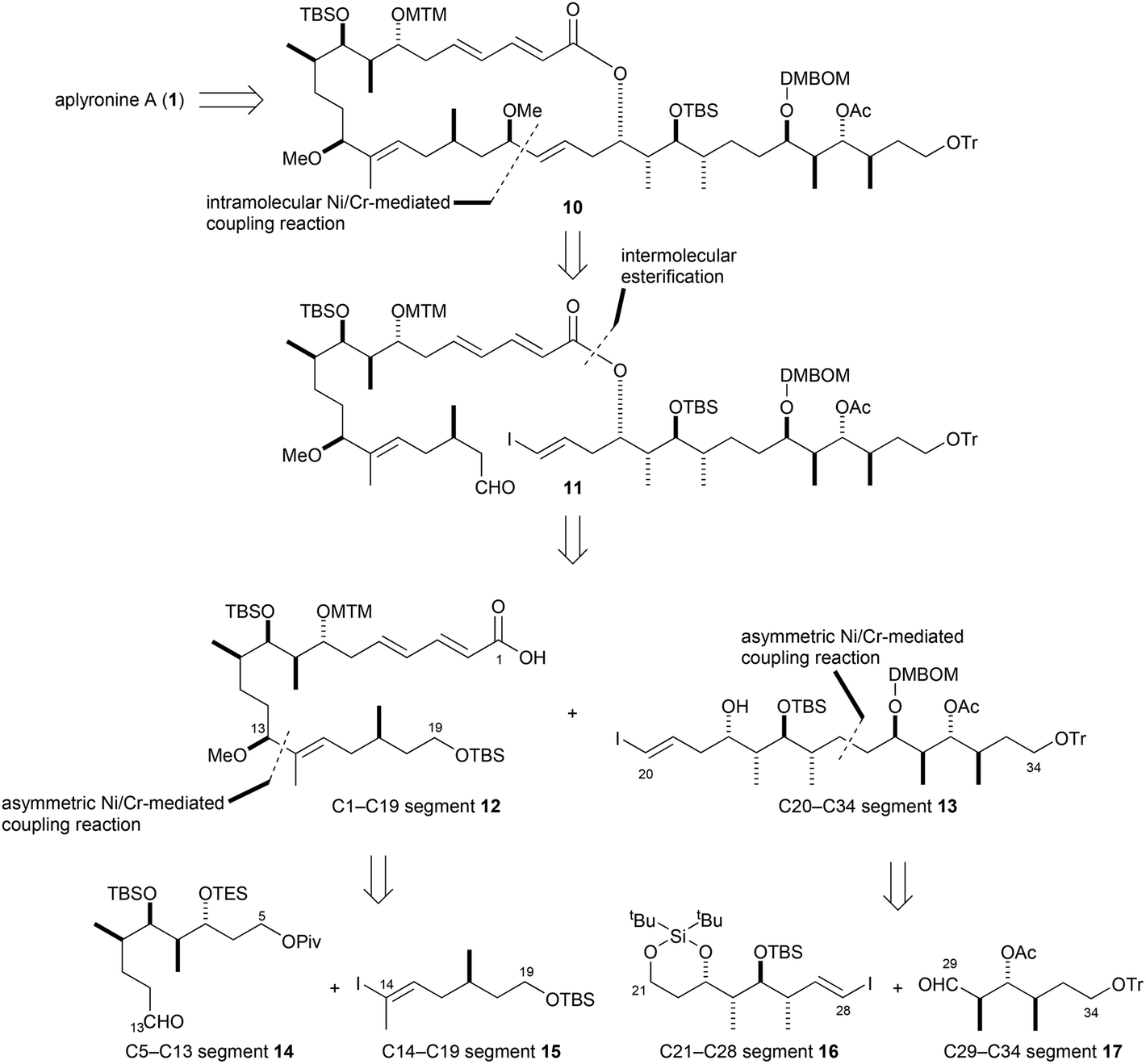
For years, radiotherapy has been an essential mode of noninvasive cancer therapy and advancements have led to life saving treatments for patients. In contrast to other conventional radiotherapies, boron neutron capture therapy (BNCT) is unique in its selective destruction of cancerous cells. BNCT is based on nuclear capture and fission reactions when nonradioactive 10B is irradiated with neutrons to yield excited 11B* which decays into high energy alpha particles and 7Li nuclei. Boron is preferentially accumulated into tumour cells though non-toxic carriers and the short length of the generated neutron beams (5-9 µm) destroys nearby cells leaving the surrounding healthy tissue intact.
The development of carrier systems that deliver sufficient amounts of boron to carry out effective destruction of all vicinal tumour cells has been a significant area of BNCT research for many years. A recent breakthrough made by Professors Atsushi Ikeda of Hiroshima University, Takeshi Nagasaki of Osaka City University and Kazuya Koumoto of Konan University has led to the development of a novel method for incorporating boron-containing moieties within hydrophobic lipid membranes.
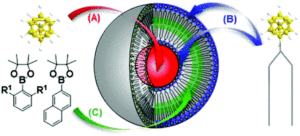
Liposomes have been studied extensively as boron carriers and are promising candidates for BNCT as lipids display low toxicity. In the past, boron-containing liposomes have been prepared by dissolution of boron compounds in an internal water phase (Figure, A) or by using amphiphilic boron compounds embedded in the liposomal bilayer such that boron coats the outer surface of the lipid membrane (Figure, B). Unfortunately, even when these two methods are used in conjunction, the concentration of boron within the liposome is insufficient for effective BNCT and increasing liposomal dosing is problematic as high concentrations of lipids interfere with uptake mechanisms in the liver.
A novel method for boron transport is therefore of high value and in their recent OBC publication, the first example of boron incoporation into liposomal lipid membranes was successfully demonstrated. Key was the preparation of aryl pinacolato boronate esters bearing methyl groups at both ortho positions (Figure, R1 positions) of the phenyl rings which helped in stabilizing the boronate esters against hydrolysis.
While the concentration of boron within the lipid membrane was not particularly high, this study has unlocked yet another avenue through which non-toxic liposomal boron carrier concentrations can be improved and when used with previous boron loading methods, produces liposomes with sufficient boron concentrations to carry out BCNT. This result will no doubt enhance BNCT and lead to critical, life saving therapies for cancer patients.
To find out more see:
Lipid-membrane-incorporated arylboronate esters as agents for boron neutron capture therapy
Masafumi Ueda, Kengo Ashizawa, Kouta Sugikawa, Kazuya Koumoto, Takeshi Nagasaki and Atsushi Ikeda
DOI:10.1039/C6OB02142E
Victoria Corless is currently completing her Ph.D. in organic chemistry with Prof. Andrei Yudin at The University of Toronto. Her research is centred on the synthesis of kinetically amphoteric molecules, which offer a versatile platform for the development of chemoselective transformations with particular emphasis on creating novel biologically active molecules.