The Organic & Biomolecular Chemistry web theme issue focussing on the exciting area of foldamer chemistry is now available for you to access, with 21 articles for you to read, 4 of which have so far appeared on the covers of issues.
Here are some of the popular articles that others have been reading:
Regio- and diastereoselective fluorination of alicyclic β-amino acids
Loránd Kiss, Enikő Forró, Santos Fustero and Ferenc Fülöp
Org. Biomol. Chem., 2011, 9, 6528-6534
DOI: 10.1039/C1OB05648D
Conformational stability of collagen triple helices functionalized in the Yaa position by click chemistry
Roman S. Erdmann and Helma Wennemers
Org. Biomol. Chem., 2012, 10, 1982-1986
DOI: 10.1039/C2OB06720J
Stereoselective preparation of β,γ-methano-GABA derivatives
David J. Aitken, Ludovic Drouin, Sarah Goretta, Régis Guillot, Jean Ollivier and Marco Spiga
Org. Biomol. Chem., 2011, 9, 7517-7524
DOI: 10.1039/C1OB06095C
Design and synthesis of trans-3-aminopyran-2-carboxylic acid (APyC) and α/β-peptides with 9/11-helix
Gangavaram V. M. Sharma, Kodeti Srinivas Reddy, Shaik Jeelani Basha, Kondreddi Ravinder Reddy and Akella V. S. Sarma
Org. Biomol. Chem., 2011, 9, 8102-8111
DOI: 10.1039/C1OB06279D
To read the rest of this great web themed issue… CLICK HERE
| What do you think if this area of chemistry? Do you have a favourite article from the collection? Let us know your thoughts by leaving a comment below. |





















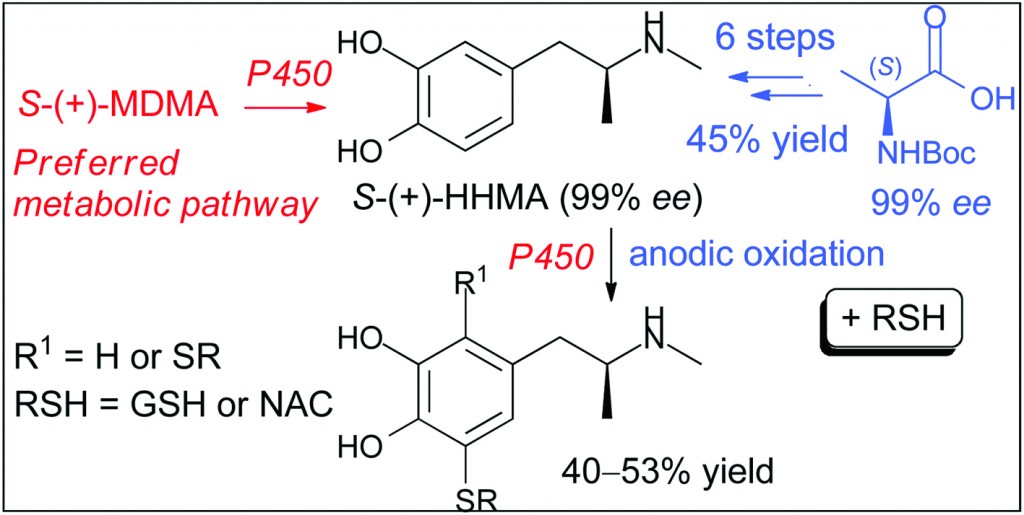 MDMA, also known as “ecstasy”, is a psychoactive drug with selective neurotoxic potential toward brain serotonin neurons. Despite intensive research, the precise mechanism by which MDMA selectively damages brain neurons in most species remains unknown. One hypothesis is that MDMA neurotoxicity may at least partially be a consequence of its metabolism. In particular, O-demethylenated MDMA metabolites such as N-methyl-α-methyldopamine (HHMA) have been postulated to serve as precursors for toxic catechol–thioether conjugates.
MDMA, also known as “ecstasy”, is a psychoactive drug with selective neurotoxic potential toward brain serotonin neurons. Despite intensive research, the precise mechanism by which MDMA selectively damages brain neurons in most species remains unknown. One hypothesis is that MDMA neurotoxicity may at least partially be a consequence of its metabolism. In particular, O-demethylenated MDMA metabolites such as N-methyl-α-methyldopamine (HHMA) have been postulated to serve as precursors for toxic catechol–thioether conjugates.