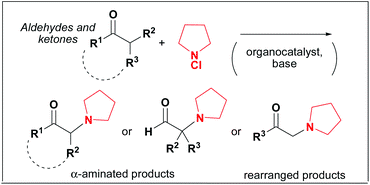
This Communication by Marco Bella et al. is a study of the historically famous Rabe amination, presenting preliminary results for a “modern” organocatalytic version of the reaction whereby direct functionalisation of ketones and aldehydes with N-chloro amines is achieved in mild conditions. Bella et al. also report that when aldehydes bearing a CH2 moiety in the α-position are used, a novel rearrangement is observed leading to α-amino ketones.
Have a look at the Communication for more details on the experimental procedure and possible mechanistic explanation for this interesting reaction, it’s free to access for 4 weeks!
The Rabe amination after a century: direct addition of N-heterocycles to carbonyl compounds
Daniele M. Scarpino Schietroma, Mattia R. Monaco, Valerio Bucalossi, Philipp E. Walte, Patrizia Gentili and Marco Bella
DOI: 10.1039/C2OB25595B














 Welcome to OBC issue 22
Welcome to OBC issue 22