The importance of natural products in health and medicine is enormous owing to their diverse biological activities and their role as a basis for drug development. Endeavours in total synthesis have attracted some of the most talented organic chemists–providing intellectual and creative outlets–and have been central to the evolution and technical development of organic synthesis.
In the early 1990’s, Professor Hideo Kigoshi of the University of Tsukuba reported the first total synthesis of the marine macrolide Aplyronine A which is still a highly desirable synthetic target due to its potent and unique biological properties.
In collaboration with Professor Ichiro Hayakawa of Okayama University, the group has recently published a highly efficient second-generation total synthesis of Aplyronine A which requires fewer synthetic steps and boasts an improved overall yield. Issues with poor stereoselectivity, regioselectivity and isomerization were overcome through the optimization of a Ni/Cr-mediated coupling.
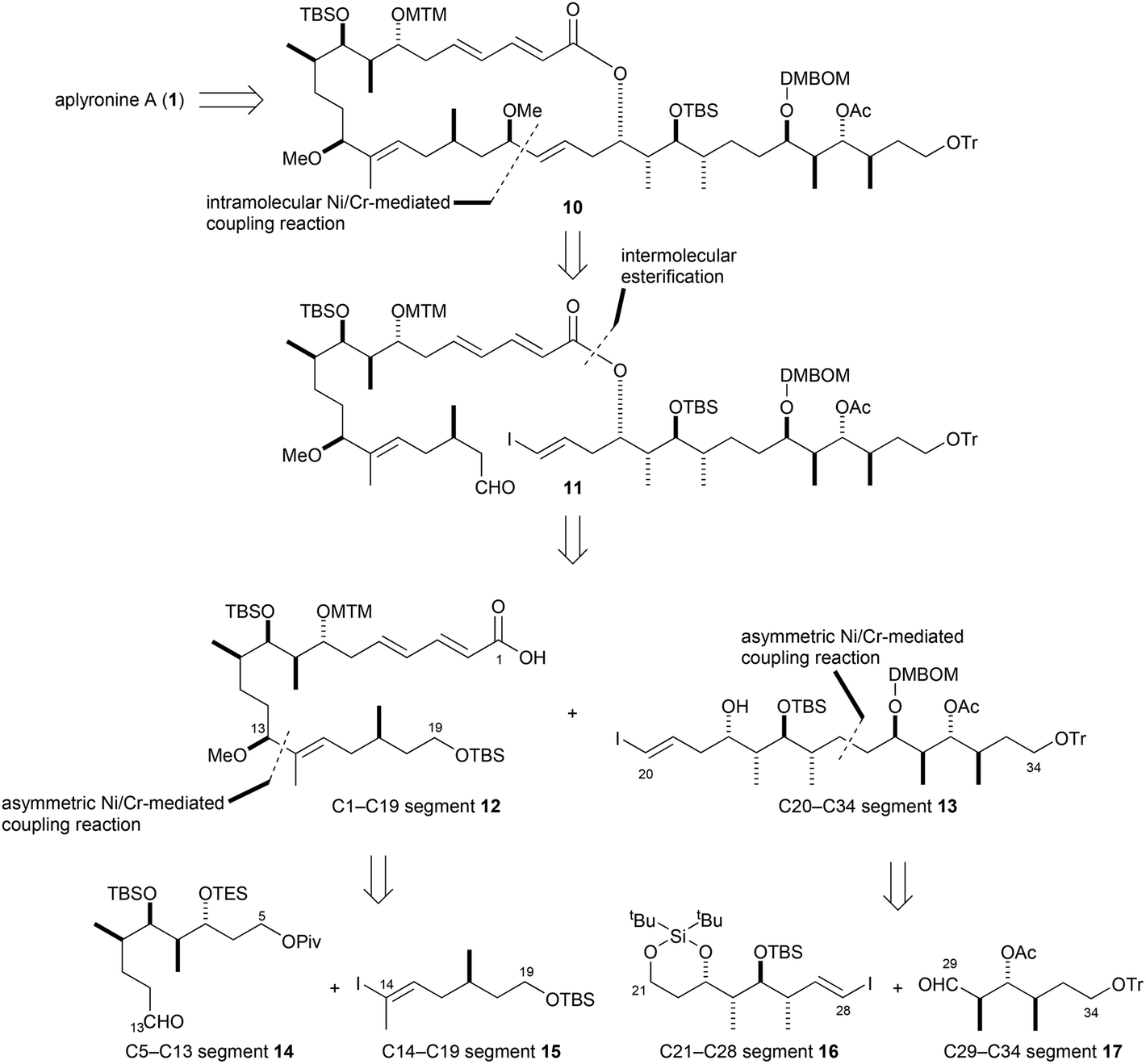
As shown in the retrosynthetic pathway below, Aplyronine A was obtained from 10, the same intermediate used in the first generation synthesis however, instead of the Julia coupling between ketone and sulfone-containing fragments, the macrolactone 11 was cyclized via an intramolecular Ni/Cr-mediated coupling. This modification drastically reduced the number of unwanted byproducts obtained from the Julia coupling and eliminated the need to employ a modified Yamaguchi lactonization which had resulted in the formation of an undesired 26-membered lactone that required an additional isomerization to yield the desired product. Precursor 11 was constructed through an intermolecular esterification between carboxylic acid 12 and alcohol 13 which were each prepared through asymmetric Ni/Cr-mediated couplings. In the case of the carboxylic acid, route efficiency was further enhanced as this strategy resulted in the simultaneous formation of the C14–C15 (E)-trisubstituted double bond and the C13 stereogenic center through the use of a chiral ligand.
In addition to establishing an efficient synthetic pathway to Aplyronine A, the Ni/Cr-mediated coupling has significant potential in the preparation of structurally diverse derivatives which may result in enhanced biological activity and the discovery of a novel lead.
The popularity of natural products as synthetic targets will continue as they provide unparalleled inspiration for drug leads and the synthesis of non-natural compounds. Strategies to develop concise and efficient synthetic routes are significantly important not only in terms of their utility in medicine but in the downstream application of novel synthetic methodologies developed during the process of their total synthesis.
To find out more see:
Second generation total synthesis of aplyronine A featuring Ni/Cr-mediated coupling reactions
Ichiro Hayakawa, Keita Saito, Sachiko Matsumoto, Shinichi Kobayashi, Ayaka Taniguchi, Kenichi Kobayashi, Yusuke Fujii, Takahiro Kanekob and Hideo Kigoshi
DOI: 10.1039/C6OB02241C
Victoria Corless is currently completing her Ph.D. in organic chemistry with Prof. Andrei Yudin at The University of Toronto. Her research is centred on the synthesis of kinetically amphoteric molecules, which offer a versatile platform for the development of chemoselective transformations with particular emphasis on creating novel biologically active molecules.