 Computational chemistry is a powerful tool for understanding real-world chemical problems. The gap between experiment and computational models is growing ever smaller. Calculated results for isolated molecules are becoming more relevant and reliable calculations for larger and larger molecular systems are becoming more accessible.
Computational chemistry is a powerful tool for understanding real-world chemical problems. The gap between experiment and computational models is growing ever smaller. Calculated results for isolated molecules are becoming more relevant and reliable calculations for larger and larger molecular systems are becoming more accessible.
A computational study of enantioselective spiroacetalization catalyzed by phosphoric acids carried out by researchers at the Universidad de Salamanca and Oxford University effectively demonstrates the ability of advanced computational methods to elucidate key and often subtle factors that lead to different reaction outcomes.
The study uses a hybrid quantum mechanics (QM)/molecular mechanics (MM) method which makes computational simulations of large systems feasible by combining an accurate quantum mechanical description of the ‘interesting’ part of the system (i.e. the catalyst active site) with the computational efficiency of molecular mechanics applied to the surroundings. This way, one can assess the importance of environmental effects while avoiding a high computational cost. This is accomplished by partitioning the system’s total energy into inner (or active) and outer parts. The interactions within the inner part are then treated with the computationally higher quantum mechanics level and the outer parts are described using less expensive, lower level molecular mechanics methods.
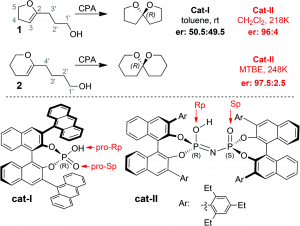 |
The origin of greater enantioselectivity for the imidodiphosphoric acid (Figure, cat-II) over the binol phorophoric acid (Figure, cat-I) was determined through exhaustive analysis of transition state conformers using the QM/MM method. Ultimately, it was determined that the source of chiral discrimination in catalyst II comes from a unique, bifunctional hydrogen bonding interaction between the catalyst and substrate. This confining interaction ends up limiting the accessible area to the imidodiphosphoric oxygen, resulting in an enantioselective outcome.
The significance of this work lies in the utility of theoretical models in explaining important empirical results. The ability to dissect a mechanism and identify the influential factors that determine a selective reaction outcome could not have been so easily accomplished without the use of computational analysis and will no doubt aid in the design of future organocatalysts for small, non-sterically demanding molecules.
To find out more see:
QM/MM study on the enantioselectivity of spiroacetalization catalysed by an imidodiphosphoric acid catalyst: how confinment works
Luis Simón and Robert S. Paton
DOI: 10.1039/C6OB00045B
Victoria Corless is currently completing her Ph.D. in organic chemistry with Prof. Andrei Yudin at The University of Toronto. Her research is centred on the synthesis of kinetically amphoteric molecules, which offer a versatile platform for the development of chemoselective transformations with particular emphasis on creating novel biologically active molecules.











