Researchers in China have developed a simple and effective way to construct spiropyrazolones, stereochemically complex and challenging structural motifs found in many biologically active molecules.
| An organocatalytic asymmetric double Michael cascade reaction of unsaturated ketones and unsaturated pyrazolones: highly efficient synthesis of spiropyrazolone derivatives Jinyan Liang, Qiao Chen, Luping Liu, Xianxing Jiang and Rui Wang DOI: 10.1039/C2OB27095A |
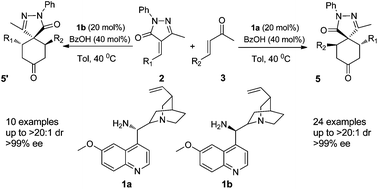
3-Pyrazolones are privileged structures and molecules that contain them have been found to possess a huge range of useful biological effects such as antibiotic and anti-tumour properties. In addition to their marked medicinal potential, they have also found use as dyes and anti-corrosives.
For these reasons, methods for the construction of pyrazolone derivatives have attracted intense interest from organic chemists in recent years. The synthesis of optically active pyrazolones, including spiropyrazolones, is particularly challenging with only a handful of syntheses reported in the current literature.
Professor Rui Wang, of the State Key Laboratory of Applied Organic Chemistry, Lanzhou University in China and his research group have been looking into new ways to make spirocyclic compounds, using organocatalytic cascade reactions.
In their latest HOT PAPER, they turn their attention to the construction of spiropyrazolones. A very elegant organocatalytic double Michael cascade reaction between unsaturated ketones and unsaturated pyrazolones yields the desired spiropyrazolone core structure in excellent diastereo- and enantioselectivities.
This reaction provides rapid entry into stereochemically complex spiropyrazolone derivatives and will no doubt prove useful in the manufacture of future medicines and materials.
Published on behalf of Annabella Newton, Organic & Biomolecular Chemistry web science writer.










