Issue 45 of Organic & Biomolecular Chemistry is now online, so hop to it and take a cheeky peek….
 Featuring in the issue this week:
Featuring in the issue this week:
Leaping on to the front cover is this image (right) from Andrew B. Holmes, Annabella F. Newton and colleagues. The cover highlights the work of Holmes et al. exploring the synthesis of the histrionicotoxin family, which includes some interesting investigations into the intriguing observed regioselectivity of the dipolar cycloaddition during the key cascade hydroxylamine-alkyne cyclisation/nitrone cycloaddition to form the azaspirocyclic core.
Intramolecular nitrone dipolar cycloadditions: control of regioselectivity and synthesis of naturally-occurring spirocyclic alkaloids
Alastair J. Hodges, Joseph P. Adams, Andrew D. Bond, Andrew B. Holmes, Neil J. Press, Stephen D. Roughley, John H. Ryan, Simon Saubern, Catherine J. Smith, Michael D. Turnbull and Annabella F. Newton
DOI: 10.1039/C2OB26333E
 The inside cover of this week’s issue (left) features the work of Feng Shi and co-workers from Henan University. Shi et al. re-examine the use of the Kobayashi benzyne precursor in the [3 + 2] cycloaddition of arynes with 3-oxidopyridinium species, for the first time in 16 years, expanding investigations further than ever before.
The inside cover of this week’s issue (left) features the work of Feng Shi and co-workers from Henan University. Shi et al. re-examine the use of the Kobayashi benzyne precursor in the [3 + 2] cycloaddition of arynes with 3-oxidopyridinium species, for the first time in 16 years, expanding investigations further than ever before.
Aryne cycloaddition with 3-oxidopyridinium species
Hailong Ren, Chunrui Wu, Xiuxiu Ding, Xiaoge Chen and Feng Shi
DOI: 10.1039/C2OB26519B
Both of these papers have been highlighted as being HOT chemistry by the reviewers, and they are both FREE to access for the next 6 weeks, so enjoy!
Also in this issue: 6 Communications and a further 14 papers full of great content.
















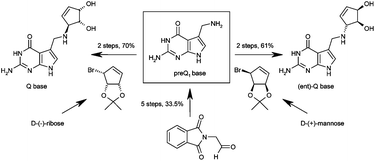
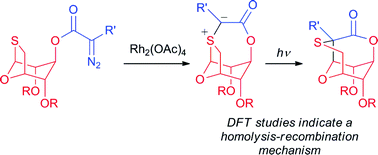
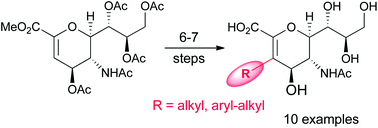
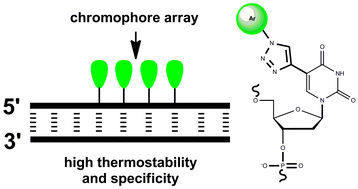
![A cucurbit[8]uril sponge](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C2OB26774H)
