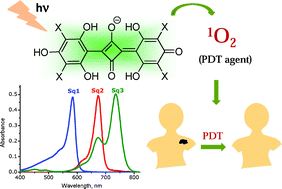
Following our latest OBC Hot Article by Prof. Bradley D. Smith (available here), Squaraines are in the spotlight again in this review article by Danaboyina Ramaiah et al..
The Perspective highlights the recent developments of squaraines as PDT sensitizers, including:
- Design principles
- Squaraines as singlet oxygen generators
- Squaraines as two-photon absorbing agents
- Carrier systems for squaraine dyes
- In vitro and in vivo studies of squaraine-PDT action
Timely and HOT, why not read this Perspective now – it will be FREE to access for the next 4 weeks
Squaraine dyes in PDT: from basic design to in vivo demonstration
Rekha R. Avirah, Dhanya T. Jayaram, Nagappanpillai Adarsh and Danaboyina Ramaiah
Org. Biomol. Chem., 2012, Advance Article
DOI: 10.1039/C1OB06588B, Perspective













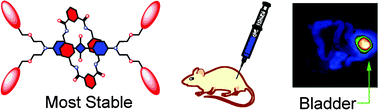
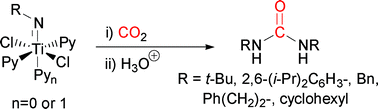
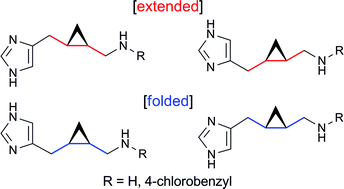
![GA[1]](https://blogs.rsc.org/ob/files/2011/12/GA12.gif)
 Welcome to
Welcome to  Areas covered include:
Areas covered include:![GA[1]](https://blogs.rsc.org/ob/files/2011/12/GA11.gif)