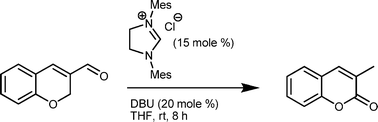
A new nucleophilic heterocyclic carbene mediated transformation of 2H-chromene-3-carboxaldehydes has been reported by Vijay Nair, CSIR, et al.
The team were attempting to make endocyclic homoenolates from 2H-chromene-3-carboxaldehyde, but instead discovered a novel transformation which resulted in 3-methyl coumarin. Coumarins have demonstrated important bioactives and therefore the group envisage that this reaction may be a useful future route to coumarin derivatives.
This hot piece of synthesis is currently free to access for four weeks:
A novel NHC-catalyzed transformation of 2H-chromene-3-carboxaldehydes to 3-methyl-2H-chromen-2-ones
Vijay Nair, C. R. Sinu, R. Rejithamol, K. C. Seetha Lakshmi and Eringathodi Suresh
Org. Biomol. Chem., 2011, Advance Article
DOI: 10.1039/C1OB05325F