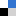The performance of nanomaterials is intricately linked to their size and combination form. Quantum dots (QDs), for instance, exhibit unique electronic and quantum properties due to their size effect. Moreover, the optical and electronic attributes of QD assemblies can be tailored by adjusting the size of individual QDs and their spatial arrangement within the assembly. Thus, it is imperative to explore suitable and universally applicable methods to prepare adjustable QDs and their assemblies.
In a recent study, Akter et al. devised mesoscopic QD assemblies using a novel bio-catalytic nanoparticle shaping (BNS) approach. Specifically, the authors employed L-lysine as a linker to assemble CdSe/CdS QDs, initially yielding ultra-large QD assemblies. Subsequently, these assemblies were catalytically cleaved by trypsin, resulting in mesoscopic QD assemblies (ms-QD) with a size of 84 nm (Fig. 1A). Relative to single QDs, the redshift and weakened emission observed in the photoluminescence spectrum of ms-QD suggest the presence of internal emission reabsorption processes within the assembly, which holds promise for leveraging energy or electron/hole transfer processes in the production of new optical materials.
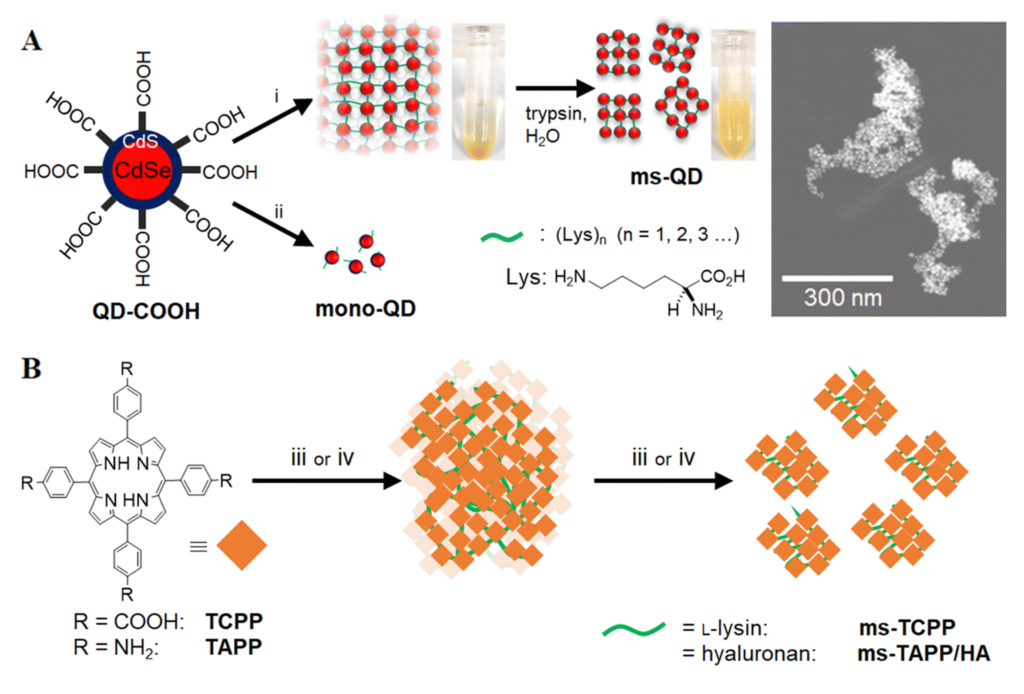
Fig 1. (A) Synthetic pathway of ms-QD by the BNS method and corresponding TEM image, and (B) the synthetic scheme of organic molecule assemblies. Reproduced from DOI: 10.1039/D4NH00134F with permission from the Royal Society of Chemistry.
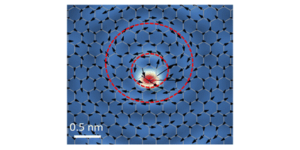
Additionally, the authors synthesized and investigated nanoassemblies of the organic molecule tetrakis(4-carboxyphenyl)porphyrin (Fig. 1B) using a similar method. They observed significant differences in reactive oxygen generation under light among the various assemblies, indicating the potential to modulate the molecular function of the particle’s core unit by altering the composition of connecting molecules.
In summary, this method demonstrates high versatility and can be employed for preparing assemblies of both QDs and organic molecules. Furthermore, it allows for the replacement of biological enzymes/substrates as needed to generate various nanoassemblies with unique physicochemical properties for further applications.
To find out more, please read:
Bio-catalytic nanoparticle shaping for preparing mesoscopic assemblies of semiconductor quantum dots and organic molecules
Rumana Akter, Nicholas Kirkwood, Samantha Zaman, Bang Lu, Tinci Wang, Satoru Takakusagi, Paul Mulvaney, Vasudevanpillai Biju and Yuta Takano
Nanoscale Horiz., 2024, Advance Article
About the blogger

Chao Wang is a postdoctoral fellow at Oregon State University and a member of the Nanoscale Horizons Community Board. His research focuses on multiple nanomedicines (especially metal-organic frameworks) and their theranostics for cancer, endometriosis and stem cell tracking. |