Drug delivery and targeted treatment of diseases is one of the prominent focus areas of recent research. Development of new therapeutic approaches involving novel drug delivery materials (e.g., nanomaterials) requires validation that these materials do not affect the existing properties of the cellular environment. Now, researchers from the Third Military Medical University (China) and National University of Singapore (Singapore) have found that DNA-nanostructure-based drug delivery vehicles do not affect the cellular environment as previously thought, but in fact aid in restoring endothelial leakiness in vascular diseases.
For proper cellular function, endothelial barriers maintain vascular permeability by which essential nutrients and oxygen reaches the target tissues. Several diseases and inflammations cause endothelial leakiness, which in turn leads to disease progress and ineffective treatments. Now, researchers use cell and mouse models of pulmonary arterial hypertension (PAH), a lung disease, to demonstrate that DNA-based therapeutic carriers can effectively restore the endothelial barrier. They developed a triangular DNA structure and loaded small-interfering RNA (siRNA) molecules that target specific disease-associated genes. In this case, the researchers targeted the Atg101 gene that causes autophagy and in turn affects endothelial leakiness. They found that the siRNA-loaded DNA carriers were taken up by cells and reduced endothelial gaps to 0.3% compared to untreated cells that showed 10% endothelial gaps, thus providing a 30-fold improvement. This treatment was specific to the siRNA cargo loaded in the structure. When they used a random siRNA sequence loaded on to the DNA structures, there was no improvement in endothelial gaps. The group then tested the siRNA-loaded DNA structures in mice and found that the drug-loaded DNA structures provided protection against right ventricular and pulmonary artery dysfunction, a promising step forward to creating a treatment strategy for such diseases.
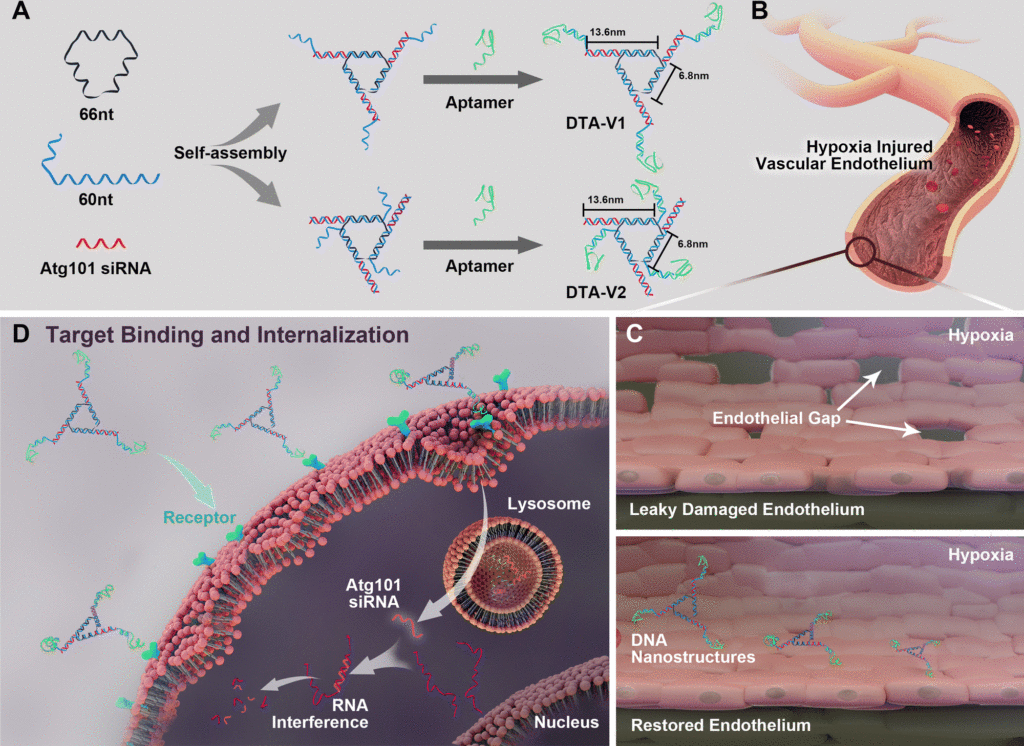
Fig. 1 (A) Design of DNA aptamer and Atg101 siRNA (siAtg101) conjugated DNA nanostructures. DNA aptamers are positioned either at the protruding points (DTA-V1) or the corners of the structure core (DTA-V2). (B) Aptamer-decorated DNA nanostructures bind to HPAECs and are subsequently internalized (C and D). The DNA nanostructures might be internalized through aptamer-mediated endocytosis. The embedded siRNA takes effect and restores endothelial integrity similar to the reversal of “NanoEL”. Reproduced from DOI: 10.1039/D2NH00348A with permission from the Royal Society of Chemistry.
This study provides new information on how nanomaterials interact with biological systems and affect cellular environment such as endothelial leakiness which is typically associated with tumor regions. As DNA structures could successfully delivery siRNA molecules to suppress endothelial leakiness related to a vascular disease, this study opens up the possibility of using DNA-based drug delivery carriers in therapeutics approaches beyond just cancer.
To find out more, please read:
Attenuating endothelial leakiness with self-assembled DNA nanostructures for pulmonary arterial hypertension
Qian Liu, Di Wu, Binfeng He, Xiaotong Ding, Yu Xu, Ying Wang, Mingzhou Zhang, Hang Qian, David Tai Leong and Guansong Wang
Nanoscale Horiz., 2023, 8, 270–278
About the blogger

Arun Richard Chandrasekaran is a Senior Research Scientist at The RNA Institute at the University at Albany, State University of New York, and member of the Nanoscale Horizons Community Board. Dr Chandrasekaran’s research lab focusses on using DNA as a material to build nanoscale structures, with applications in drug delivery, data storage and crystallography. You can follow Arun on Twitter @arunrichardc |