On the cover of Issue 11 is a hot article from Mark Bradley and coworkers on a method to track innate immune cell migration in vivo using dye-labelled peptoids.
On the cover of Issue 11 is a hot article from Mark Bradley and coworkers on a method to track innate immune cell migration in vivo using dye-labelled peptoids.
Far red and NIR dye-peptoid conjugates for efficient immune cell labelling and tracking in preclinical models
Kevin Dhaliwal, Géraldine Escher, Asier Unciti-Broceta, Neil McDonald, A. John Simpson, Chris Haslett and Mark Bradley
Med. Chem. Commun., 2011, 2, 1050-1053
DOI: 10.1039/C1MD00171J
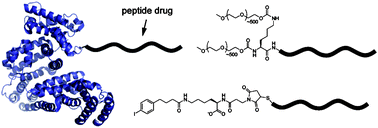
Also in this issue is Ying-Wei Yang’s review on biocompatible nanovalves for drug delivery and release:
Towards biocompatible nanovalves based on mesoporous silica nanoparticles
Ying-Wei Yang
Med. Chem. Commun., 2011, 2, 1033-1049
DOI: 10.1039/C1MD00158B











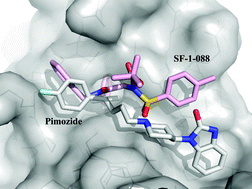 This mini-review from
This mini-review from 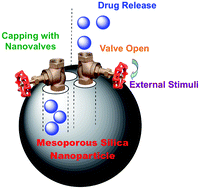

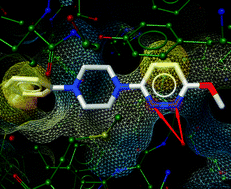
 The p53 signalling pathway is responsible for regulating the cell cycle, initiating DNA repair and triggering apoptosis where necessary. Its crucial importance can clearly be seen by the fact that about half of human cancers contain alterations in the p53 pathway. The proteins MDM2 and MDMX (a.k.a. MDM4) negatively regulate p53, and altered levels of these proteins are often deemed responsible for p53 alterations. As a result MDM2 has been extensively studied, but few MDM2 inhibitors have made it as far as clinical trials.
The p53 signalling pathway is responsible for regulating the cell cycle, initiating DNA repair and triggering apoptosis where necessary. Its crucial importance can clearly be seen by the fact that about half of human cancers contain alterations in the p53 pathway. The proteins MDM2 and MDMX (a.k.a. MDM4) negatively regulate p53, and altered levels of these proteins are often deemed responsible for p53 alterations. As a result MDM2 has been extensively studied, but few MDM2 inhibitors have made it as far as clinical trials.