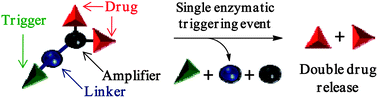
 In this Hot article, scientists from France present a self-immolative dendritic glucuronide prodrug designed for the release of two doxorubicin molecules after a single triggering event.
In this Hot article, scientists from France present a self-immolative dendritic glucuronide prodrug designed for the release of two doxorubicin molecules after a single triggering event.
‘While numerous glucuronide prodrugs have been studied so far, the efficiency of this targeting strategy is limited by the poor turnover of beta-glucuronidase in malignant tissues. Our novel dendritic prodrug which is programmed to release two molecules of doxorubicin after a single enzymatic activation step may permit to overcome this drawback’, the authors explain.
The biological experiments conducted have shown that this novel enzyme-responsive dendritic prodrug is twice more toxic than its monomeric counterpart against H661 lung cancer cells. It is hoped that this novel approach may open new avenues in selective cancer chemotherapy.
Selected as HOT, read this article for free now.
A self-immolative dendritic glucuronide prodrug of doxorubicin
Marion Grinda, Jonathan Clarhaut, Brigitte Renoux, Isabelle Tranoy-Opalinski and Sébastien Papot
Med. Chem. Commun., 2011, Advance Article
DOI: 10.1039/C1MD00193K, Concise Article











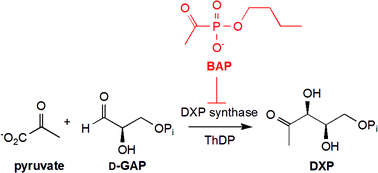

 This mini-review from
This mini-review from ![GA[9]](https://blogs.rsc.org/md/files/2011/10/GA9-300x118.gif)